Method for preparing T cells with knockout of double genes TCR and HLA
A double-gene, cell-based technology, applied to cells modified by introducing foreign genetic material, recombinant DNA technology, using vectors to introduce foreign genetic material, etc., can solve the problem of limiting the use of peripheral blood mononuclear cells, the number, activity and proliferation of T cells Limited capacity, loss of treatment options, etc., to achieve the effect of facilitating tumor treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1C
[0031] Implementation case 1 CRISPR / Cas9-TCR and CRISPR / Cas9-HLA vector construction
[0032] 1. Recombinant expression vector
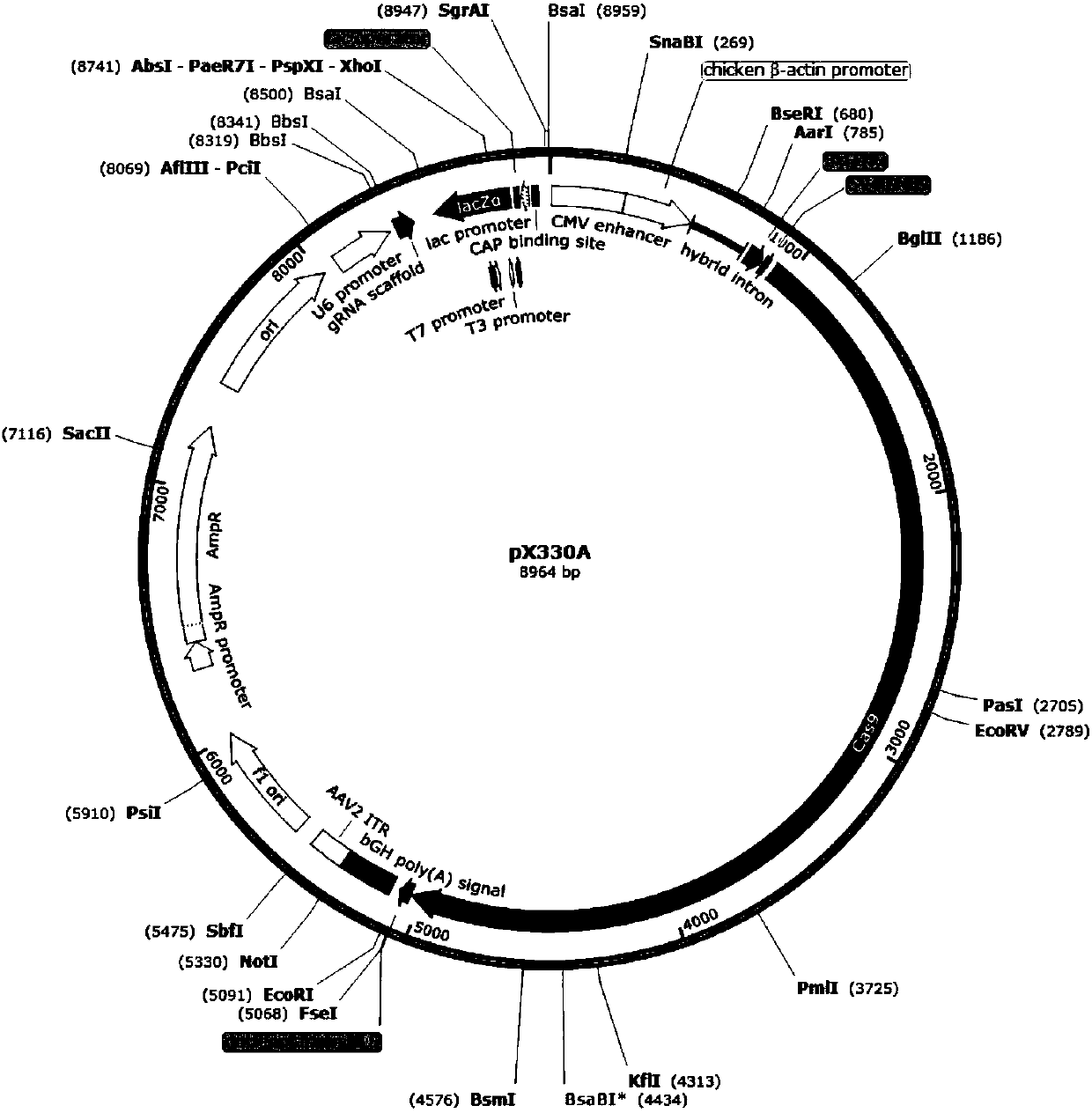
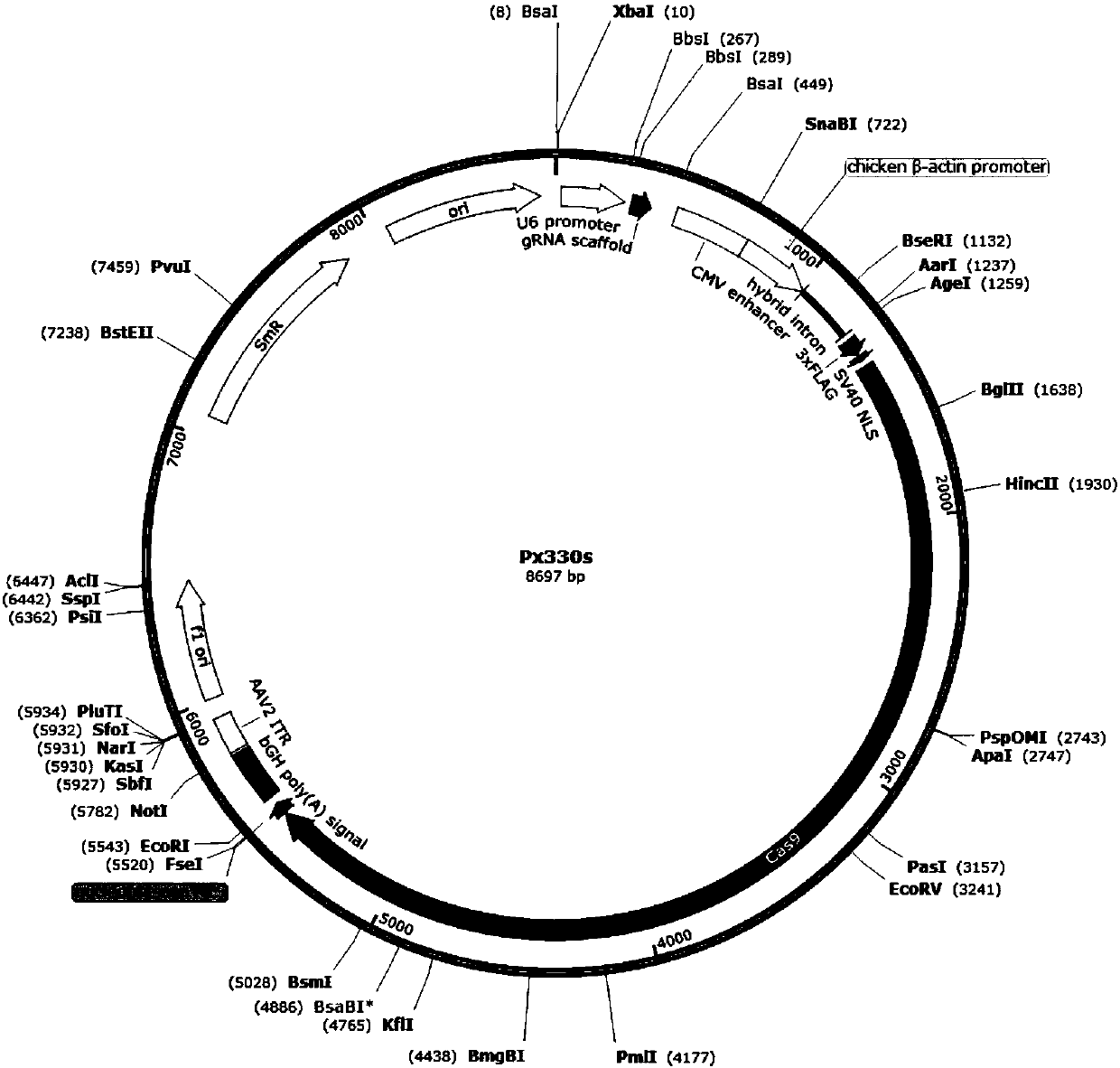
[0033] The vectors used in the present invention are pX330A and pX330S (the map is as follows figure 1, shown in 2), the pX330A expression gene vector contains an original replicon, a cytomegalovirus promoter sequence (CMV), and restriction endonuclease sites (BbsI, Bsa I, etc.), resistance screening gene (anti ampicillin); pX330S is similar in structure to pX330A, and the differences are mainly in the different enzyme cutting sites and the different resistance selection genes (spectinomycin). The artificially synthesized sgRNA gene is connected with the linearized backbone carrier DNA under the action of T4 ligase to form a recombinant vector.
[0034] 2. Synthesis of TRAC-sgRNA, TRBC2-sgRNA and B2M-sgRNA
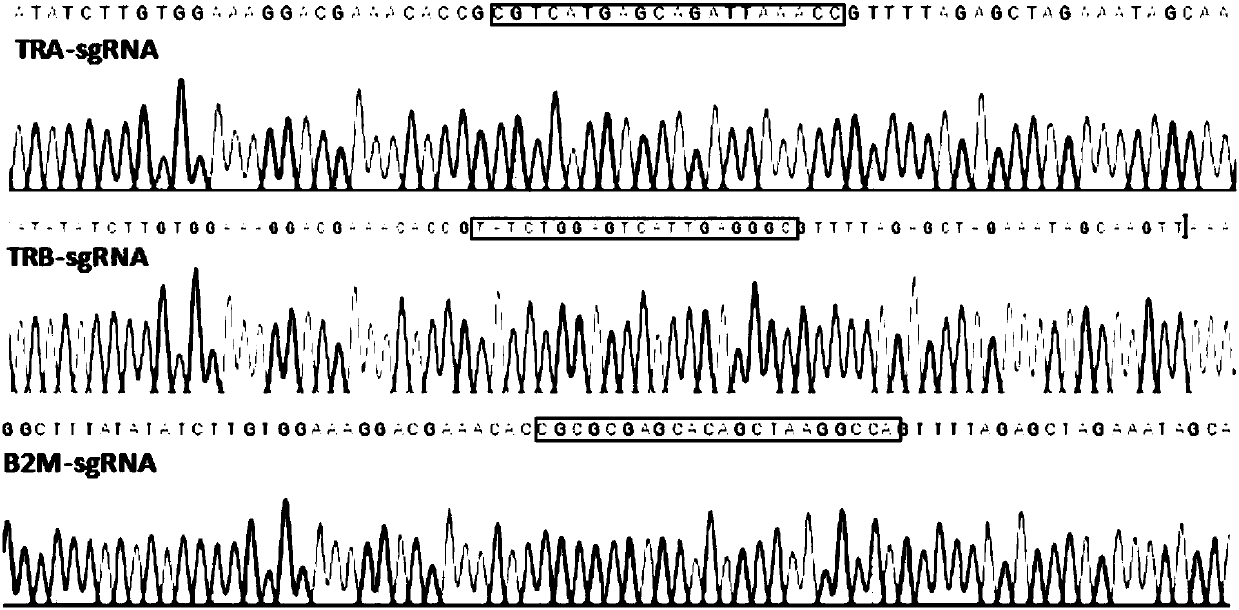
[0035] The sgRNA of TRAC, TRBC2 and B2M is located in the exon of the gene (SEQ ID NO.1~3), and the target sequence on the gene is unique. Ad...
Embodiment example 2T
[0040] Implementation Case 2 Acquisition of TCR and HLA Double Gene Knockout T Cells
[0041] Fresh peripheral blood mononuclear cells were separated by density gradient, activated by CD3 and CD28 monoclonal antibodies, cultured with IL-2 (final concentration 300IU / ml), activated into T cells, and then the recombinant vector CRISPR / Cas9 prepared in Example 1 -HLA and CRISPR / Cas9-TCR plasmids were co-transfected into T cells, and TCR and HLA double gene knockout was performed. Transfection has been called:
[0042] Add the plasmids (CRISPR / Cas9-TCR, CRISPR / Cas9-HLA) into the electroporation cuvette respectively, each electroporation plasmid is 200ng, add cells (1×10 7 ), invert the electric shock cup to make it evenly mixed, put the electric shock cup into the electric shock tank, pulse electric shock once (2.0KV, 25uFD), then transfer the cells to the well containing 0.5ml DMEM medium, shake the well gently After 24h-48h of electroporation, the gene was transiently expressed...
Embodiment example 3T
[0045] Implementation case 3 TCR and HLA double gene knockout T cells combined with CAR to treat tumors
[0046] The above isolated TCR and HLA double gene knockout T cells were infected by lentivirus with the CAR for the treatment of different types of tumors. The specific process is: Leader-scFv-CD8-CD137-CD3ζ-T2A-HSV-TK nucleotide Synthesis, the gene fragment of the synthetic Leader-scFv-CD8-CD137-CD3ζ-T2A-HSV-TK is inserted into the NotI-AsiSI site of the pLent-C-GFP vector, after the sequencing is correct, a plasmid is constructed, and the plasmid Transfect 293T cells, package into a lentivirus carrying the encoding gene, add 1 mL of the lentivirus carrying the Leader-scFv-CD8-CD137-CD3ζ-T2A-HSV-TK encoding gene to culture TCR and HLA double gene knockout T cells (1×10 7 ) in a petri dish, mix well, and replace with fresh culture medium after 24 hours.
[0047] Further, after the infection is completed, the CAR-expressing TCR and HLA double-gene knockout T cells are exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com