Red fluorescent powder and preparation method thereof
A technology for red phosphor powder and phosphor powder is applied in the field of red phosphor powder and its preparation, and can solve the problems of difficult preparation, short lifespan, and difficulty in powder mixing of high-efficiency power UVLEDs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The present invention also provides a method for preparing the above-mentioned fluorescent powder, comprising: A) mixing Ca precursor, Ti precursor and Mn precursor, performing high-temperature solid-state reaction to obtain fluorescent powder;
[0035] The molar ratio of Ca, Ti and Mn in the Ca precursor, Ti precursor and Mn precursor is 1:(1-x):x; 0<x<1.
[0036] Wherein, the x is the same as that described above, and will not be repeated here.
[0037] The Ca precursor is a compound containing Ca well known in the art, and there is no special limitation. In the present invention, Ca carbonate, Ca oxide, Ca oxalate and Ca nitrate are preferred. One or more of, more preferably a carbonate of Ca; the Ti precursor is an oxide of Ti; the Mn precursor is a carbonate of Mn, an oxide of Mn, an oxalate of Mn and One or more of Mn nitrates, more preferably Mn oxides.
[0038] The purity of the Ca precursor, Ti precursor and Mn precursor is preferably not less than 99.5% inde...
Embodiment 1
[0047] Raw material is CaCO 3 (analytical pure), TiO 2 (99.99%) and MnO 2(Analytical pure), the molar ratio is 1:0.98:0.02, the above raw materials are ground and mixed, dried, pressed into tablets under a pressure of 2 MPa, put into a crucible, and sintered in a high-temperature furnace at 1200°C under an ammonia reducing atmosphere After 4 hours, it was cooled to room temperature with the furnace, and the theoretical chemical composition was obtained as CaTi 0.98 mn 0.02 o 3 of fluorescent powder.
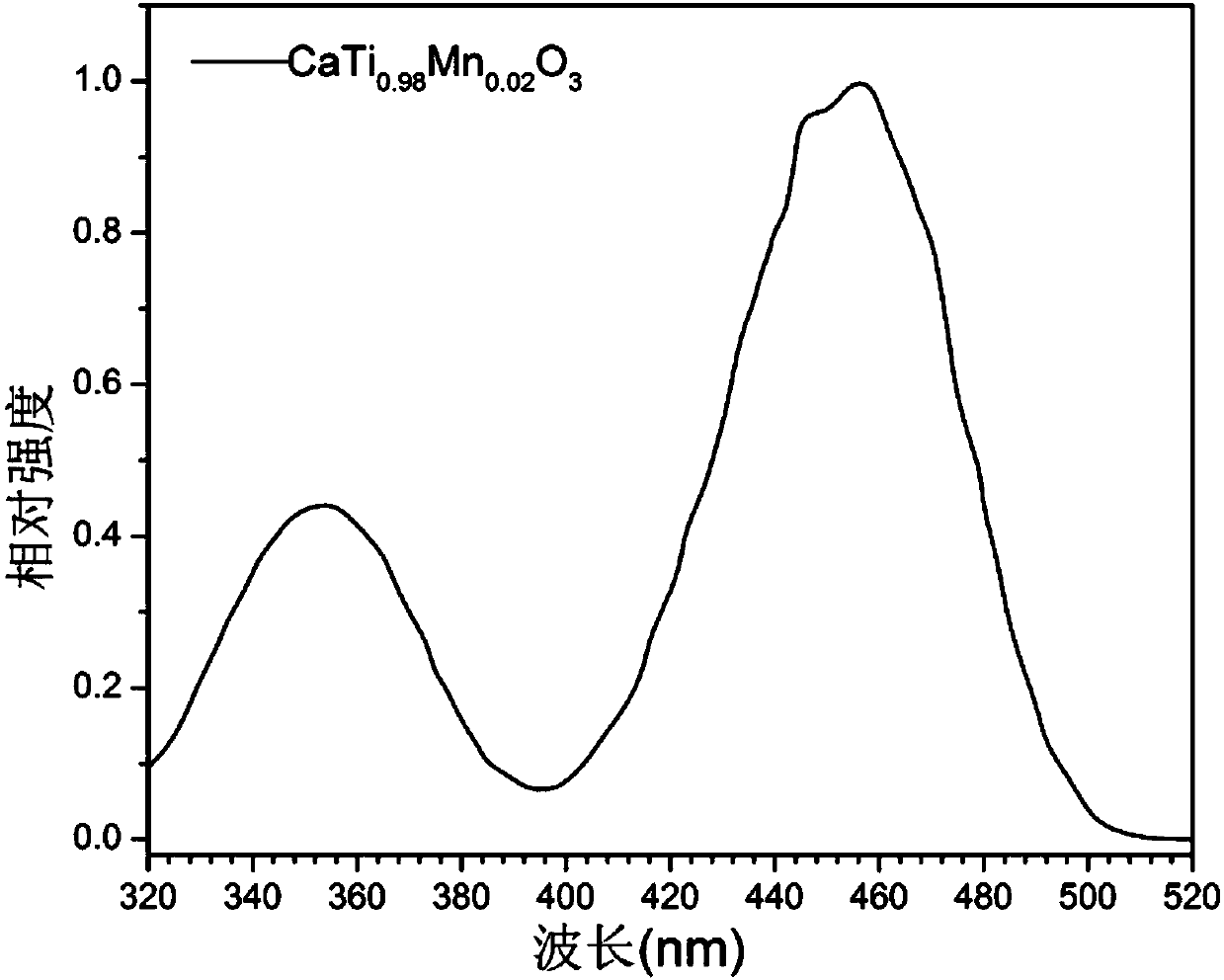
[0048] Utilize fluorescence spectrometer to analyze the fluorescent material obtained in embodiment 1, obtain its excitation spectrogram, as figure 1 shown. It can be seen that the excitation band of the fluorescent material mainly falls in the blue light region.
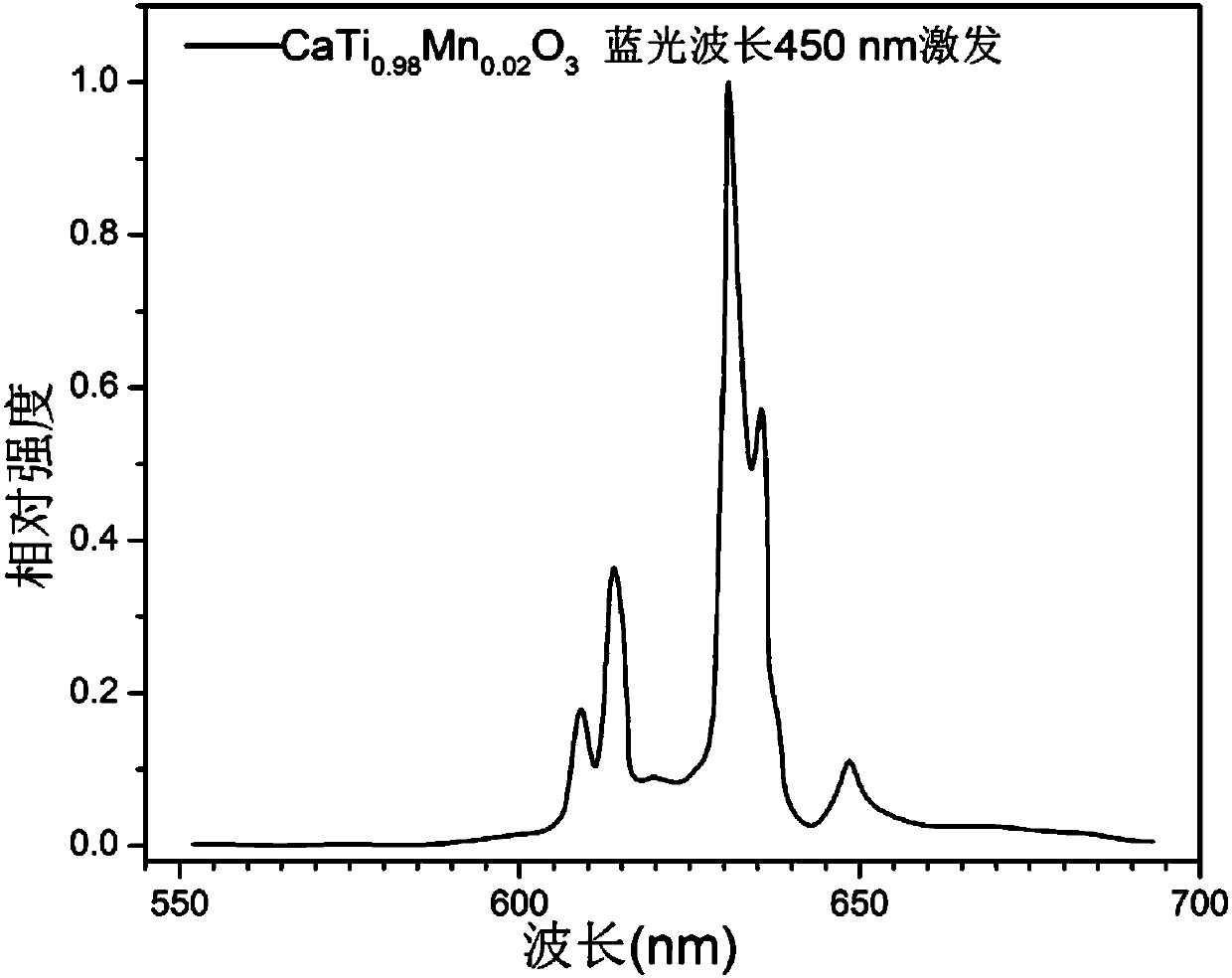
[0049] Utilize fluorescence spectrometer to analyze the fluorescent material obtained in embodiment 1, obtain its emission spectrogram, as figure 2 shown. It can be seen that the material can be effectively e...
Embodiment 2
[0051] Raw material is CaCO 3 (analytical pure), TiO 2 (99.99%) and MnO 2 (Analytical pure), the molar ratio is 1:0.95:0.05, the above raw materials are ground and mixed, dried, pressed into tablets under a pressure of 1 MPa, put into a crucible, and sintered in a high-temperature furnace at 1300°C under an ammonia reducing atmosphere After 6 hours, cool down to room temperature with the furnace, and the theoretical chemical composition is CaTi 0.95 mn 0.05 o 3 of fluorescent powder.
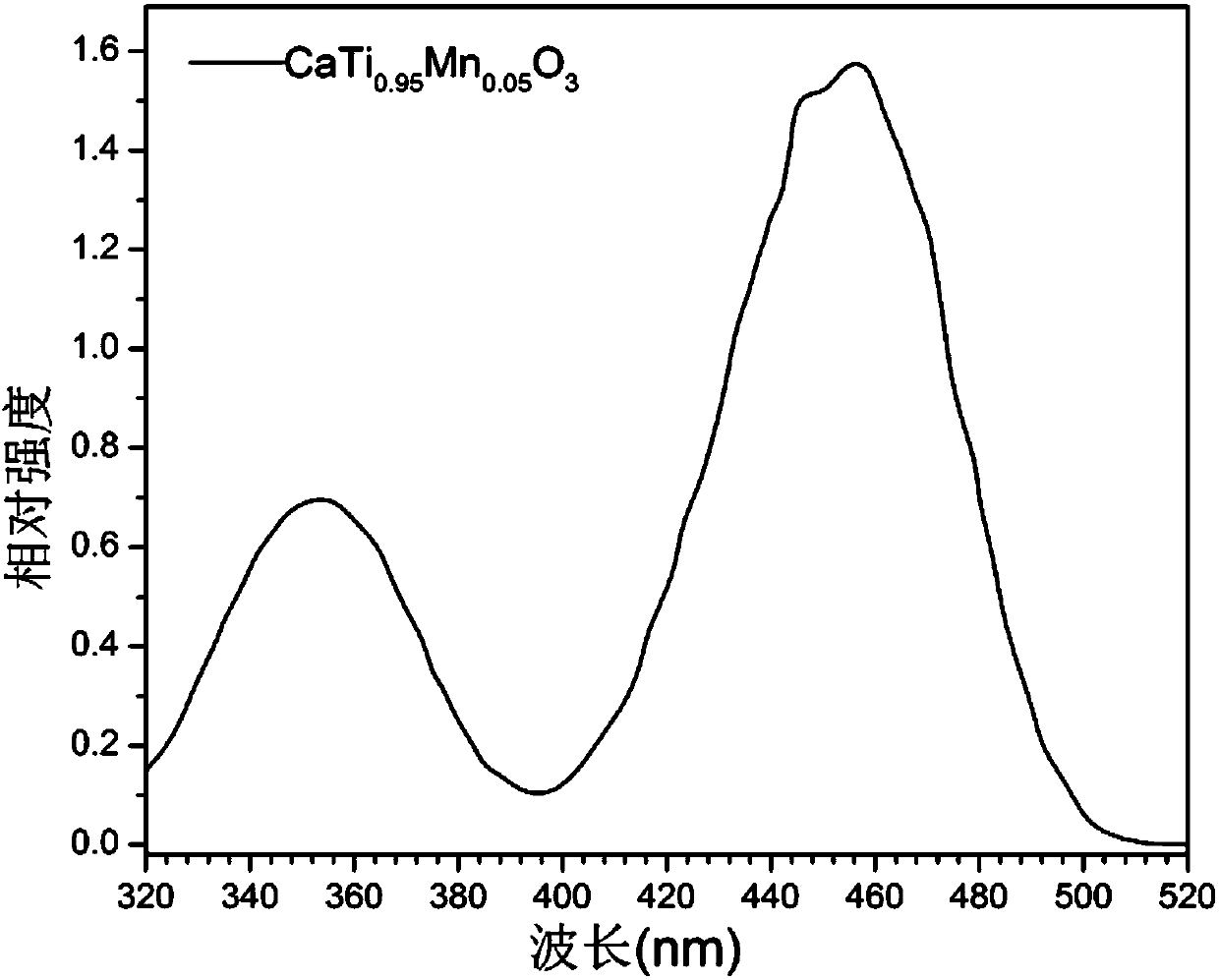
[0052] Utilize fluorescence spectrometer to analyze the fluorescent material obtained in embodiment 2, obtain its excitation spectrogram, as image 3 shown. It can be seen that the excitation band of the phosphor mainly falls in the blue light region.
[0053] Utilize fluorescence spectrometer to analyze the fluorescent material obtained in embodiment 2, obtain its emission spectrogram, as Figure 4 shown. It can be seen that the phosphor can be effectively excited by blue light to emit...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap