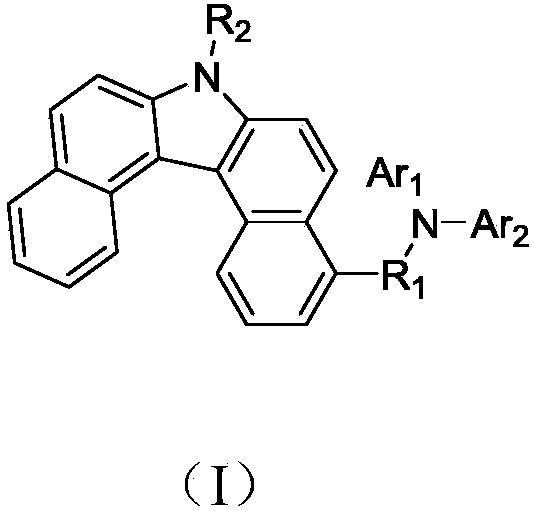
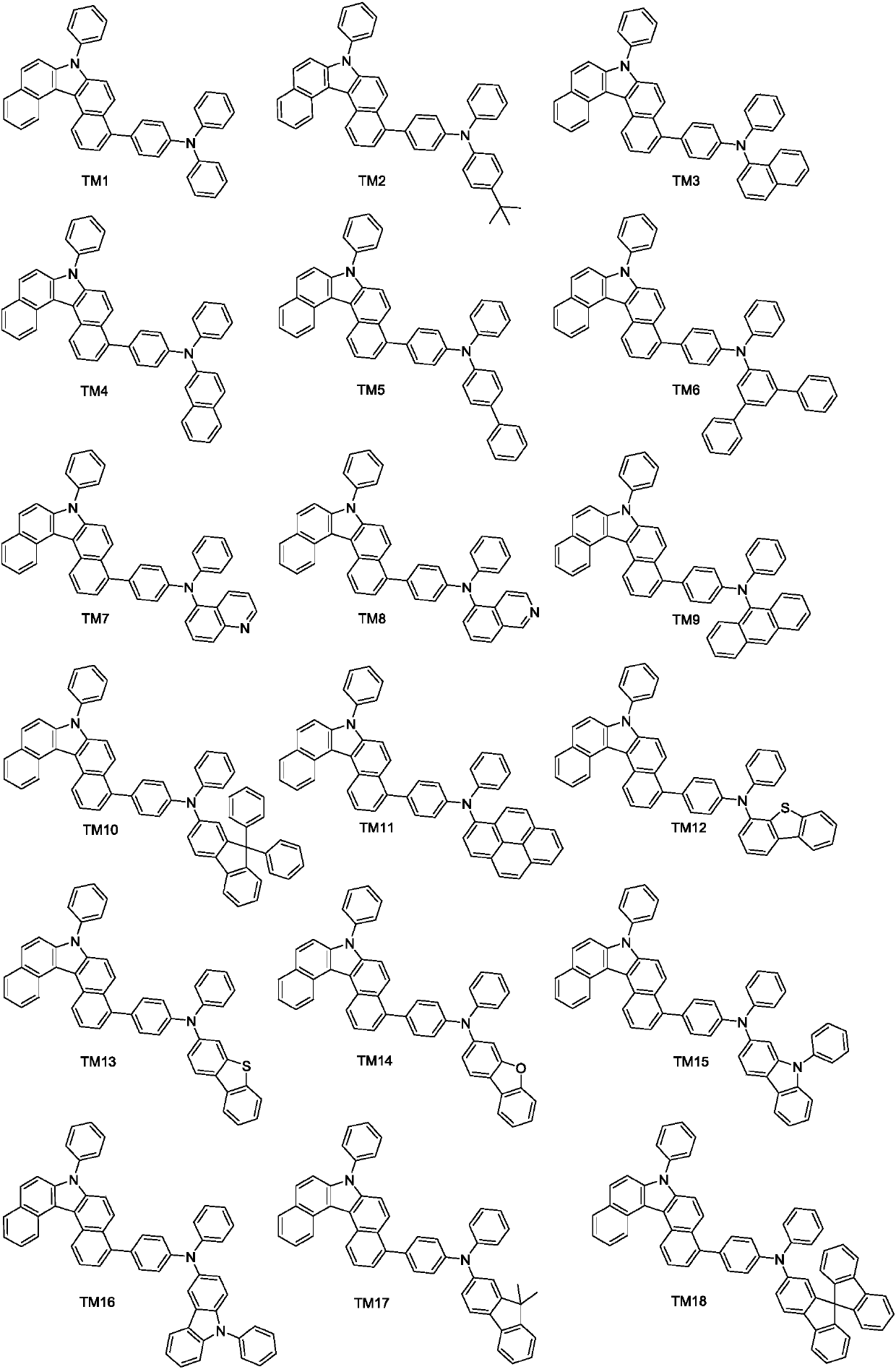
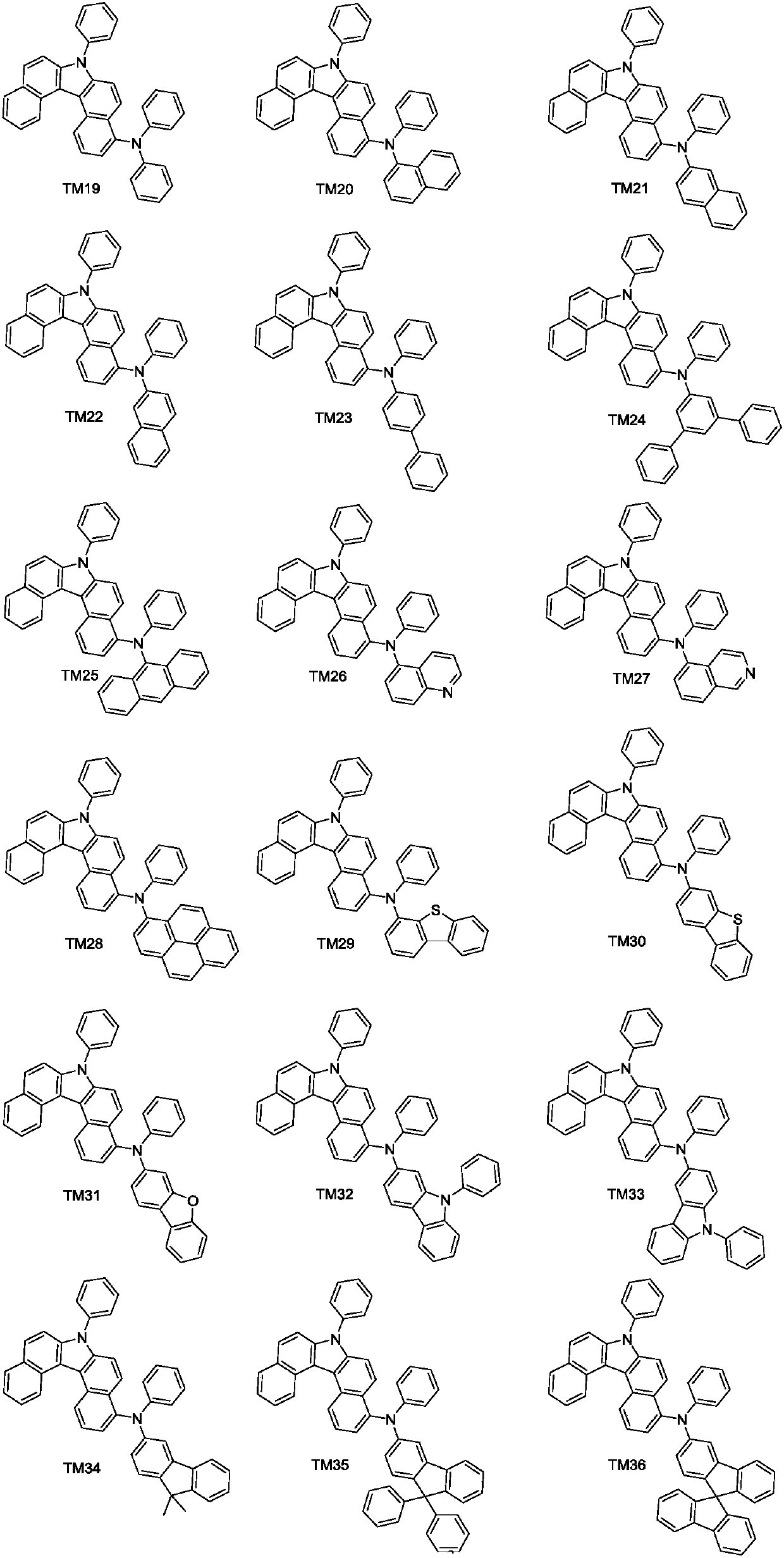
Derivative containing benzo-carbazole structure, preparation method of derivative and organic electroluminescence device
An electroluminescent device, benzocarbazole technology, applied in the field of organic photoelectric materials, can solve the problems that cannot be improved, and the luminous efficiency is reduced.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Preparation of compound TM1
[0047]
[0048] Preparation of 1-3
[0049] Under the protection of nitrogen, compound 1-1 (6.89g, 31.75mmol), 1-2 (9.08g, 31.75mmol), potassium carbonate (4.39g, 31.75mmol), and toluene 200mL were added into a 2L reactor and stirred. The temperature in the reactor was raised to 70°C, and Pd(PPh 3 ) 4 (1.04g, 0.90mmol), stirred with 100mL distilled water, stirred and refluxed for 11h, fully reacted. After adding 70mL of distilled water to stop the reaction, filter under reduced pressure, wash the solid with distilled water, and then use acetone, toluene, THF to recrystallize. After the solid is obtained, sublimate and recrystallize from toluene to obtain 9.08g of 1-3 with a yield of 75.60%.
[0050] Preparation of 1-4
[0051] In a 250ml three-necked flask, add 1-3 (9.08g, 31.75mmol), the solvent o-dichlorobenzene, and 200ml triethyl phosphite, and heat the solution to 150°C for 15h. The solvent and excess triethyl phosphite were di...
Embodiment 2
[0061] Preparation of Compound TM7
[0062]
[0063] R in Example 1 1 , R 2 and Ar 1 、Ar 2 The group is replaced by R as indicated above 1 , R 2 and Ar 1 、Ar 2 Group, other steps are the same as in Example 1 to obtain compound TM3. Mass Spectrum m / z: 637.25 (calculated: 637.26).
Embodiment 3
[0065] Preparation of compound TM12
[0066]
[0067] R in Example 1 1 , R 2 and Ar 1 、Ar 2 The group is replaced by R as indicated above 1 , R 2 and Ar 1 、Ar 2 Group, other steps are the same as in Example 1 to obtain compound TM12. Mass Spectrum m / z: 1110.40 (calculated: 1110.41).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com