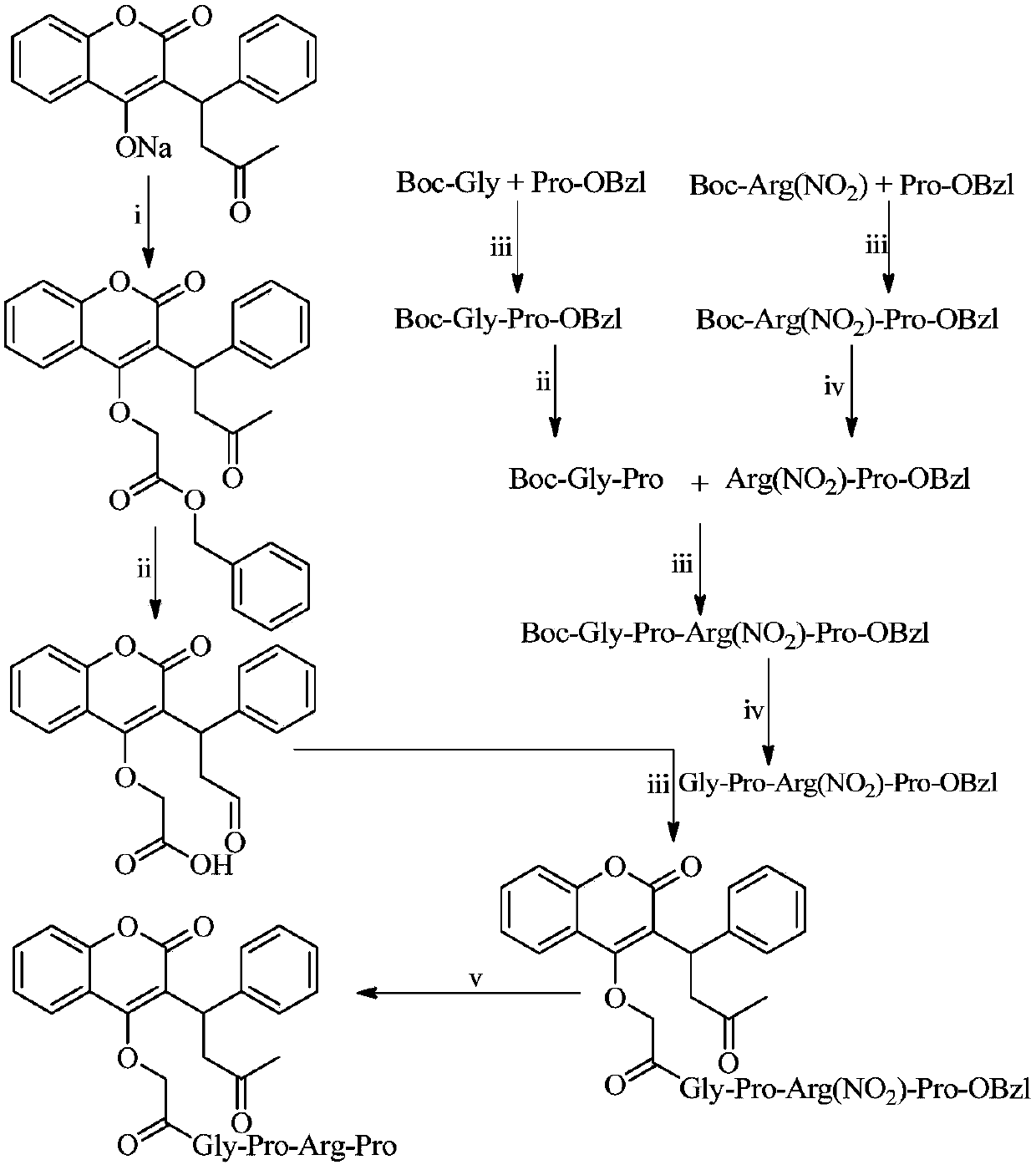
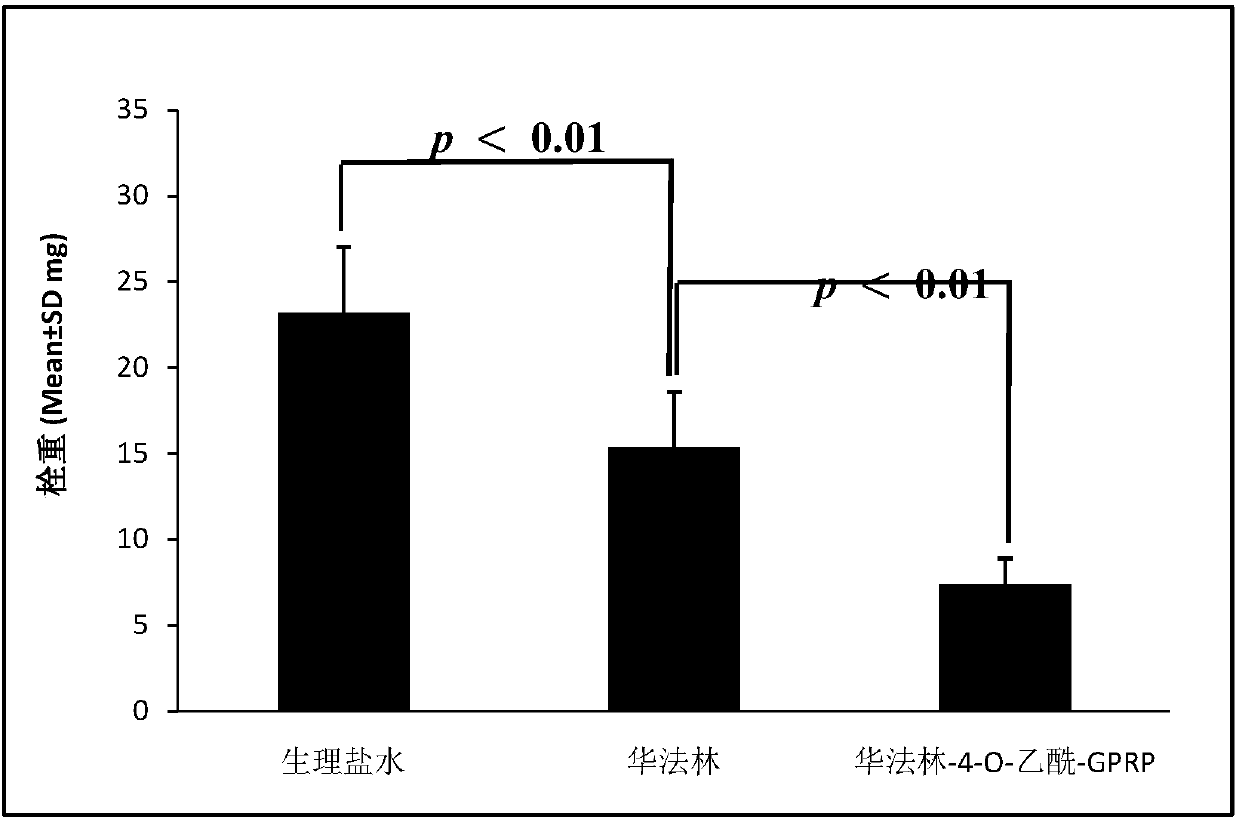
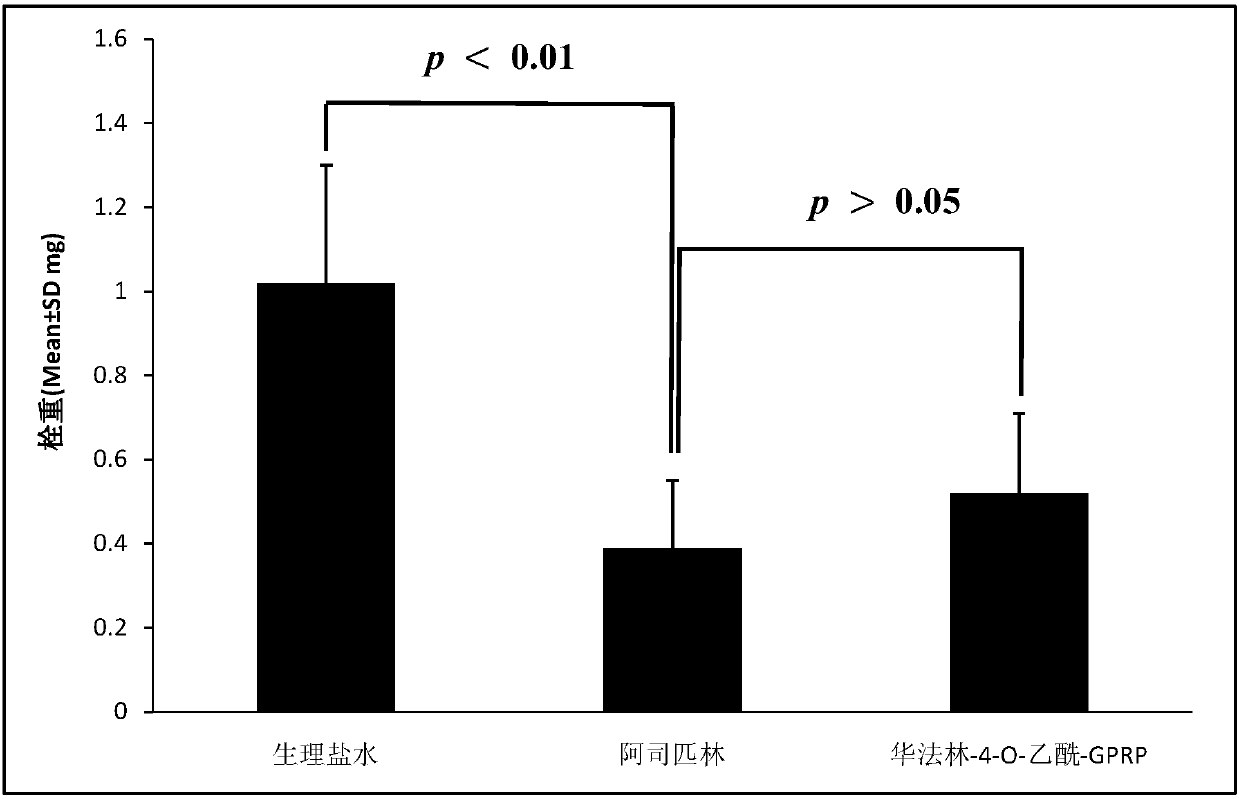
Warfarin-4-O-acetyl-GPRP and synthesis, activity and application thereof
A kind of technology of warfarin and acetyl, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 prepares warfarin-4-O-acetic acid benzyl ester
[0021] Put 6.62g (20.00mmol) of warfarin in a 250mL eggplant bottle, add about 70mL of acetone, but it cannot be completely dissolved, heat and stir in an oil bath at 45°C until warfarin dissolves, add 3.50mL (22.00mmol) of bromoacetic acid Benzyl ester, continue to react under 45°C oil bath. After reacting for 72 hours, thin layer chromatography (TLC, petroleum ether / ethyl acetate=2:1) monitored the reaction process, and warfarin had basically disappeared, and the colorless solid produced in the reaction was removed by filtration, and the filtrate was evaporated under reduced pressure to remove acetone. A pale yellow oil was obtained, which was purified by silica gel column chromatography (petroleum ether / ethyl acetate=8:1) to obtain 5.4 g (59%) of the title compound as a colorless solid. ESI-MS(m / e):457[M+H] + ; 1 H-NMR (300MHz, DMSO-d 6 )δ / ppm=7.89(dd,J 1 =3.0Hz,J 2 =9.0Hz,1H),7.63(dt,J 1 =3.0Hz,J...
Embodiment 2
[0022] Embodiment 2 prepares warfarin-4-O-acetic acid
[0023] Dissolve 4.01g (8.79mmol) of warfarin-4-O-benzyl acetate in 50mL of methanol, add 405mg of Pd / C, evacuate the air in the water pump while stirring, and inject hydrogen, repeat this operation 4 times, Introduce hydrogen and stir at room temperature for 30 h. The completion of the reaction was monitored by TLC, palladium on carbon (Pd / C) was removed by filtration, and the solvent was evaporated from the filtrate under reduced pressure to obtain 2.74 g (85.2%) of the title compound as a colorless solid. ESI-MS(m / e):367[M+H] + ; 1 H-NMR (300MHz, DMSO-d 6 ):δ / ppm=12.86(s,1H),7.90(d,J=6.0Hz,1H),7.63(t,J=6.0Hz,1H),7.43~7.34(m,4H),7.27(t, J=9.0Hz, 2H), 7.17(t, J=9.0Hz, 1H), 4.99(t, J=9.0Hz, 1H), 4.75(q, J 1 =15.0Hz,J 2 =30.0Hz, 2H), 3.54~3.47(m, 2H), 2.14(s, 3H).
Embodiment 3
[0024] Embodiment 3 prepares Boc-R (NO 2 )-P-OBzl
[0025] 3.509g (11.00mmol) Boc-R (NO 2 )-OH was added to a 250mL eggplant bottle, dissolved in 80mL dry tetrahydrofuran, and 1.485g (11.00mmol) HOBt was added in an ice bath (0°C). After stirring for 10 minutes, 2.632g (12.19mmol) of DCC was added to activate 30min. A colorless solid precipitated out of the reaction solution. Add 2.516g (10.41mmol) HCl Pro-OBzl to the reaction solution under ice-cooling, adjust the pH value to 9 with N-methylmorpholine, stir and react at room temperature for 12h, then TLC (dichloromethane / methanol=20 : 1) monitor the reaction process, the raw material point disappears, filter out the colorless solid in the reaction solution, the filtrate is evaporated under reduced pressure to remove the solvent, the residue is dissolved with 100mL ethyl acetate, and the insolubles are filtered out, and the filtrate is washed with saturated NaHCO 3 solution (30mL×3), saturated NaCl solution (30mL×3), 5% KH...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com