High thermal conductivity hydrated salt phase change material and preparation method thereof
A technology of phase change materials and hydrated salts, applied in the direction of heat exchange materials, chemical instruments and methods, etc., can solve the problems of reducing the heat storage performance of phase change materials and other performance degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] This embodiment provides a high thermal conductivity hydrated salt phase change material, the preparation method of which comprises the following steps:
[0057] Preparation of reduced graphene aqueous dispersion: Prepare 2mg / mL graphene oxide aqueous dispersion by hummers method; hydrazine hydrate, polyvinylpyrrolidone and graphene oxide aqueous dispersion are magnetically stirred at 400rpm for 30min to disperse evenly; then disperse at 90°C After reacting in a constant temperature box for 2 hours, ultrasonically disperse for 30 minutes to obtain an aqueous dispersion of reduced graphene. Wherein, the mass ratio of hydrazine hydrate to the graphene oxide aqueous dispersion is 2.3:100, and the mass ratio of polyvinylpyrrolidone to the graphene oxide aqueous dispersion is 18.2:100. In this embodiment, polyvinylpyrrolidone is added as a surfactant to improve the dispersion stability of the reduced graphene aqueous dispersion.
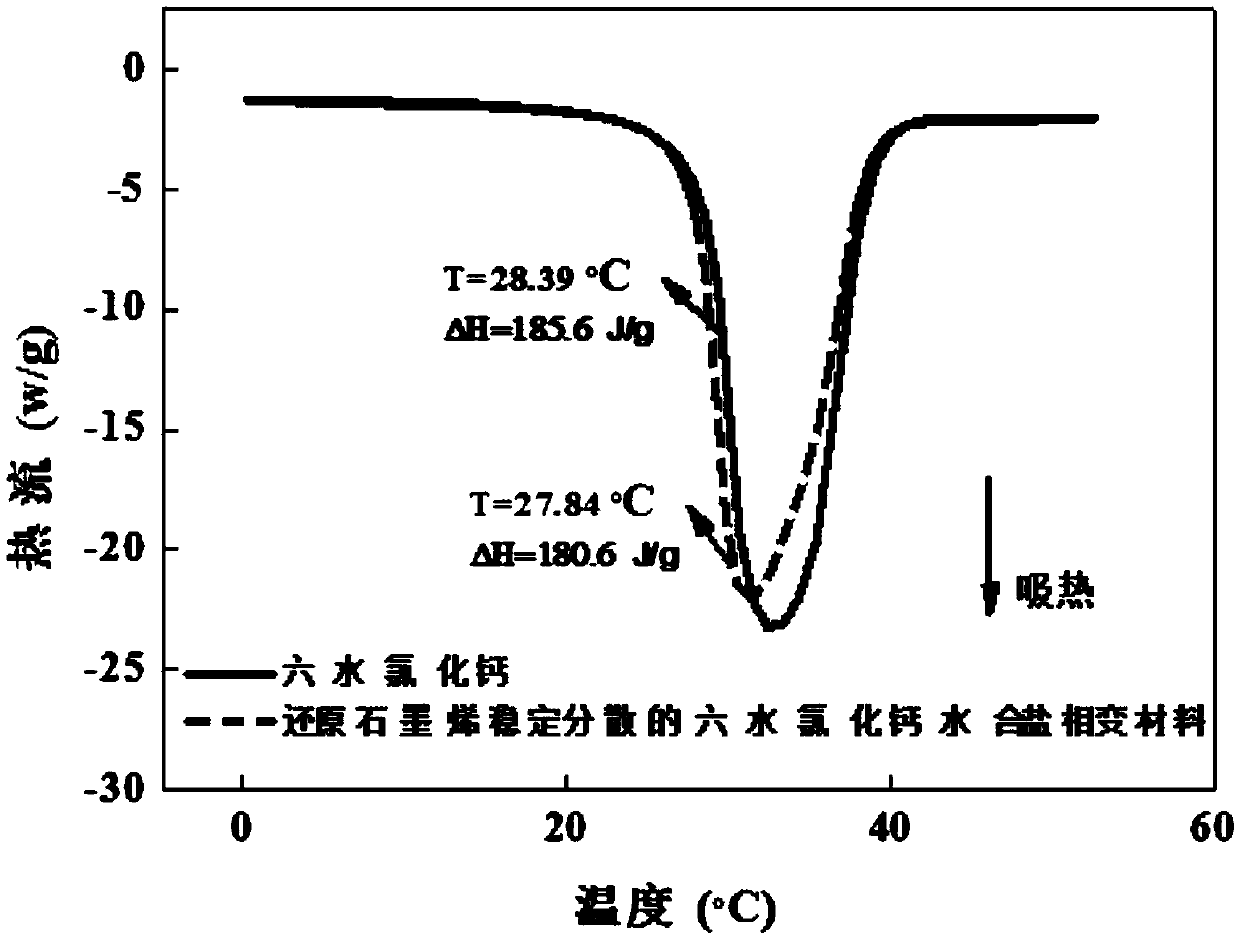
[0058] The prepared reduced graphene aqueou...
Embodiment 2
[0065] This embodiment provides a high thermal conductivity hydrated salt phase change material, the preparation method of which comprises the following steps:
[0066]Preparation of reduced graphene aqueous dispersion: Prepare 4 mg / mL graphene oxide aqueous dispersion by hummers method; hydrazine hydrate, polyvinylpyrrolidone and graphene oxide aqueous dispersion are magnetically stirred at 200 rpm for 50 min to disperse uniformly; then disperse at 110 °C After reacting in a constant temperature box for 3 hours, ultrasonically disperse for 50 minutes to obtain an aqueous dispersion of reduced graphene. Wherein, the mass ratio of hydrazine hydrate to the graphene oxide aqueous dispersion is 3:100, and the mass ratio of polyvinylpyrrolidone to the graphene oxide aqueous dispersion is 25:100. In this embodiment, polyvinylpyrrolidone is added as a surfactant to improve the dispersion stability of the reduced graphene aqueous dispersion.
[0067] The prepared reduced graphene aqu...
Embodiment 3
[0070] This embodiment provides a high thermal conductivity hydrated salt phase change material, the preparation method of which comprises the following steps:
[0071] Preparation of reduced graphene aqueous dispersion: prepare 2.5mg / mL graphene oxide aqueous dispersion by hummers method; hydrazine hydrate, polyvinylpyrrolidone and graphene oxide aqueous dispersion are magnetically stirred at 500rpm for 10min to disperse evenly; then disperse at 80 After reacting in an incubator at ℃ for 1 hour, ultrasonically disperse for 10 minutes to obtain an aqueous dispersion of reduced graphene. Wherein, the mass ratio of ethylenediamine to the graphene oxide aqueous dispersion is 1:100, and the mass ratio of polyvinylpyrrolidone to the graphene oxide aqueous dispersion is 10:100. In this embodiment, polyvinylpyrrolidone is added as a surfactant to improve the dispersion stability of the reduced graphene aqueous dispersion.
[0072] The prepared reduced graphene aqueous dispersion was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com