Method For Producing Iodine Pentafluoride
A technology of iodine pentafluoride and its manufacturing method, which is applied in the direction of interhalogen compounds, etc., can solve problems such as heat generation and difficulty in controlling the reaction, and achieve the effect of increasing the production speed and simplifying the production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
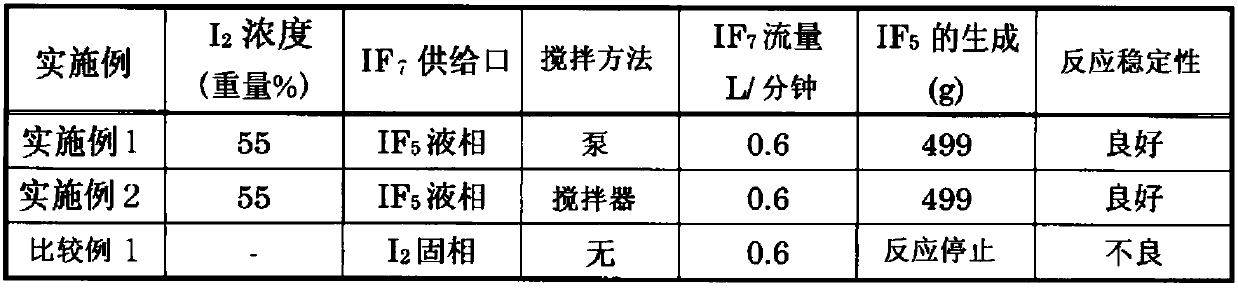
[0090] Such as figure 1 As shown, liquid iodine pentafluoride 13 with a mass of 3036 g was added to a stainless steel reaction tank 11 with a volume of 2.3 L in which the atmosphere in the tank was replaced with nitrogen, and then solid iodine 12 with a mass of 3710 g was added. The concentration of iodine 12 in liquid iodine pentafluoride 13 at the time of charging was about 55% by weight relative to the weight of iodine pentafluoride 13 and solid iodine 12 combined. As a method of stirring iodine pentafluoride 13 including solid iodine 12 in the liquid phase 15 , the pump 19 is driven to circulate the liquid phase 15 . Supply iodine heptafluoride gas from iodine heptafluoride supply source 16 to liquid phase 15 with a flow rate of 0.6L / min, make iodine heptafluoride gas react with solid iodine 12 in iodine pentafluoride 13 to obtain pentafluoride iodine. During the reaction, the pressure in the reaction vessel 11 was maintained at 93 kPa (absolute pressure). Moreover, it...
Embodiment 2
[0092] As a stirring method, instead of circulating the liquid phase 15 using the pump 19 performed in Example 1, the liquid phase 15 was stirred by rotating the stirrer 20 equipped with a rotating blade at a rotation speed of 100 rpm, and stirring the liquid phase 15 in the same manner as in Example 1 except that Iodine fluoride gas reacts with solid iodine-12. The newly generated amount of iodine pentafluoride was 499 g except for the input before the start of the experiment.
[0093] By reacting iodine in iodine pentafluoride with iodine heptafluoride by stirring with either a pump or a stirrer, heat can be effectively removed, and iodine pentafluoride can be safely and stably produced.
Embodiment 3
[0111] Iodine heptafluoride was synthesized using iodine pentafluoride obtained in Example 1. The specific manufacturing steps are as follows.
[0112] Nickel fluoride (NiF 2 ) (purity 99%, manufactured by Apollo Scientific Limited) was made into pellets (size, 4mm×4mm×2mm). 48 g (0.5 mol) of granular nickel fluoride was filled in a nickel bright annealing tube (22.1 mm inner diameter, 0.3 m length) equipped with an electric heater and a pressure gauge used as a reactor. By heating the bright annealing tube with an electric heater, the temperature of the aforementioned particles used as fillers was brought to 270°C. At this temperature, fluorine (F 2 ) and iodine pentafluoride (IF 5 ) of mixed gas (molar ratio (F 2 / IF 5 )=30.3(F 2 Concentration 96.8% by volume, IF 5 Concentration 3.20% by volume)), and discharged from the other end (outlet).
[0113] At this time, the pressure in the bright annealing tube is set to 66.7kPa (absolute pressure), and the mixed gas is 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com