A preparation process and application of β-carboline compound for preparing anti-renal fibrosis drug and/or anti-chronic kidney disease drug
A technology for chronic kidney disease and preparation technology, which is applied in the field of preparation of β-carboline compounds, and can solve problems such as harsh reaction conditions, unsatisfactory preparation methods, and low yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
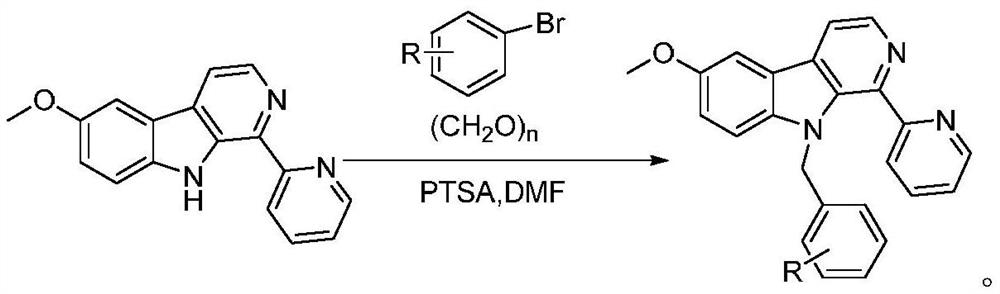
[0026] Preparation of 1-pyridine-6-methoxy-9-(2-methylbenzyl)-β-carboline
[0027] 1) 2.75g (10mmol) of 1-pyridine-6-methoxy-β-carboline, 2.22g (13mmol) of 2-methylbromobenzene and 0.52g (3mmol) of p-toluenesulfonic acid were mixed and dissolved in N,N - in dimethylformamide, heated to 80°C and stirred for 0.5 to 1 hour to obtain mixture A;
[0028] 2) Keep at 80°C, add 1.2g (40mmol) of paraformaldehyde into the mixture A obtained in step 1) in 3 to 6 times, heat up to 110°C after addition, continue stirring and contacting for 8 hours, after the reaction is complete, let it stand for cooling to room temperature, poured into saturated sodium bicarbonate, extracted with ethyl acetate, washed with water, concentrated under reduced pressure, recrystallized from dichloromethane / n-hexane (1:10), and dried to obtain 1-pyridine-6-methoxy-9- (2-Methylbenzyl)-β-carboline 3.52g, yield 92.7%, purity 99.41% (HPLC area normalization method). 1 HNMR (300MHz, DMSO-d 6 ): δ8.66(d, 1H), 8.52...
Embodiment 2
[0030] Preparation of 1-pyridine-6-methoxy-9-(3-methylbenzyl)-β-carboline
[0031] 1) 2.75g (10mmol) of 1-pyridine-6-methoxy-β-carboline, 2.57g (15mmol) of 3-methylbromobenzene and 0.52g (3mmol) of p-toluenesulfonic acid were mixed and dissolved in N,N - in dimethylformamide, heated to 70°C and stirred for 0.5 to 1 hour to obtain mixture A;
[0032] 2) Keep at 70°C, add 0.9g (30mmol) of paraformaldehyde into the mixture A obtained in step 1) in 3 to 6 times, heat up to 120°C after addition, continue stirring and contacting for 10 hours, after the reaction is complete, let it stand for cooling to room temperature, poured into saturated sodium bicarbonate, extracted with ethyl acetate, washed with water, concentrated under reduced pressure, recrystallized from dichloromethane / n-hexane (1:10), and dried to obtain 1-pyridine-6-methoxy-9- (3-methylbenzyl)-β-carboline 3.48g, yield 91.8%, purity 99.22% (HPLC area normalization method). 1 HNMR (300MHz, DMSO-d 6 ): δ8.65(d, 1H), 8.5...
Embodiment 3
[0034] Preparation of 1-pyridine-6-methoxy-9-(4-methoxybenzyl)-β-carboline
[0035] 1) 2.75g (10mmol) of 1-pyridine-6-methoxy-β-carboline, 2.24g (12mmol) of 4-methoxybromobenzene and 0.69g (4mmol) of p-toluenesulfonic acid were mixed and dissolved in N, In N-dimethylformamide, heated to 70°C and stirred for 0.5-1 hour to obtain mixture A;
[0036] 2) Keep at 70°C, add 0.6g (20mmol) of paraformaldehyde into the mixture A obtained in step 1) in 3 to 6 times, heat up to 100°C after addition, continue stirring and contacting for 8 hours, after the reaction is complete, let it stand for cooling to room temperature, poured into saturated sodium bicarbonate, extracted with ethyl acetate, washed with water, concentrated under reduced pressure, recrystallized from dichloromethane / n-hexane (1:10), and dried to obtain 1-pyridine-6-methoxy-9- (4-Methoxybenzyl)-β-carboline 3.62g, yield 91.5%, purity 99.46% (HPLC area normalization method). 1 HNMR (300MHz, DMSO-d 6)δ8.74~8.69(m, 1H), 8.5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com