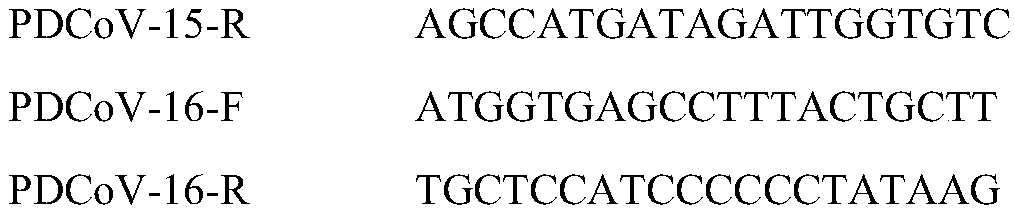Separation of porcine deltacoronavirus strain, preparation and application of inactivated vaccine
A coronavirus and inactivated vaccine technology, which is applied in the field of animal virology and zoonosis, can solve the problem of pathogenicity that has not been studied in depth, and achieve the effect of wide application range, good immunogenicity and good safety performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the acquisition of porcine delta coronavirus PDCoV CHN-HN-2014 strain
[0019] 1. Collection and processing of disease materials:
[0020]The contents of the small intestine were collected from piglets with severe diarrhea that were submitted to a pig farm in Henan Province, China, diluted with 10 times the volume of DMEM medium, fully homogenized and shaken, and centrifuged at 4200×g for 10 minutes at 4°C. The supernatant was sterilized by filtration with a 0.45-micron microporous membrane, and then used as an inoculum and frozen at -70°C for later use.
[0021] 2. RT-PCR detection of disease materials:
[0022] Get 200 microliters of the inoculum obtained in step 1, use the Trizol method (the kit is purchased from Treasure Bioengineering Dalian Co., Ltd., and operate according to the kit instructions) to extract total RNA, and use the RNA PCR Kit to reverse transcribe the extracted total RNA into After the cDNA was amplified by PCR, the upstream and do...
Embodiment 2
[0033] Embodiment 2: the application of porcine delta coronavirus CHN-HN-2014 strain in the preparation of porcine delta coronavirus inactivated vaccine:
[0034] 1. Take PDCoV CHN-HN-2014 strain (preservation number: CCTCC NO: V201650) and inoculate it into LLC-PK1 cells (purchased from ATCC cell bank, USA). TCID 50 Assay method Determine virus titer and adjust virus content to 10 8.0 TCID 50 / ml, inactivated with formaldehyde at a final concentration of 0.1%, mixed with the same quality of Montanide ISA 201 (purchased from SEPPIC, France) adjuvant, fully stirred and emulsified under sterile conditions, the emulsification temperature is 31 ° C, emulsified The time is 15 minutes. After the emulsification is complete, it is aseptically subpackaged and sealed for future use.
[0035] (1) Safety inspection of porcine delta coronavirus inactivated vaccine
[0036] Test method: choose 6 healthy piglets of age of 3-5 days, each piglet intramuscularly injects 2ml of porcine delta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com