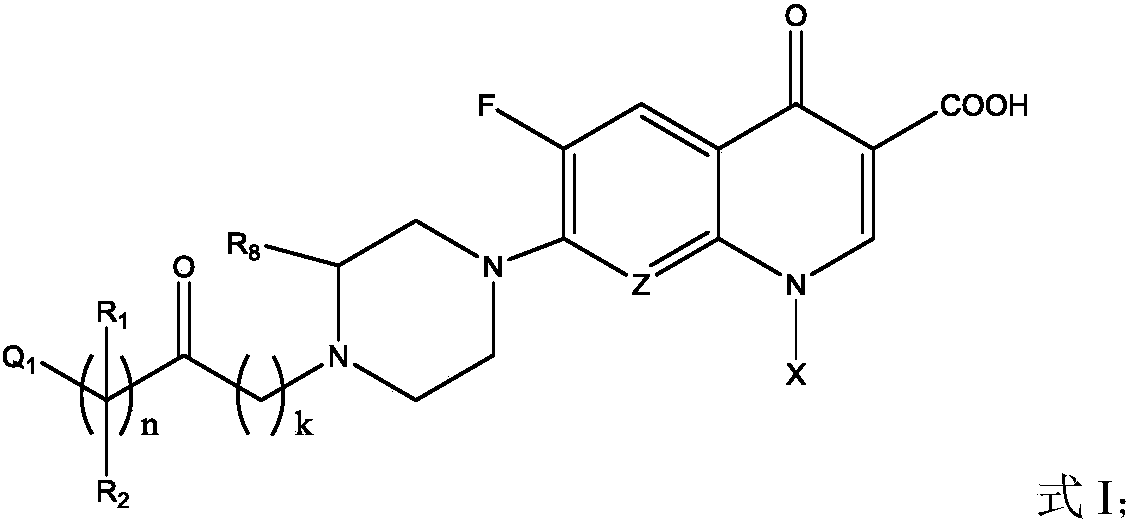
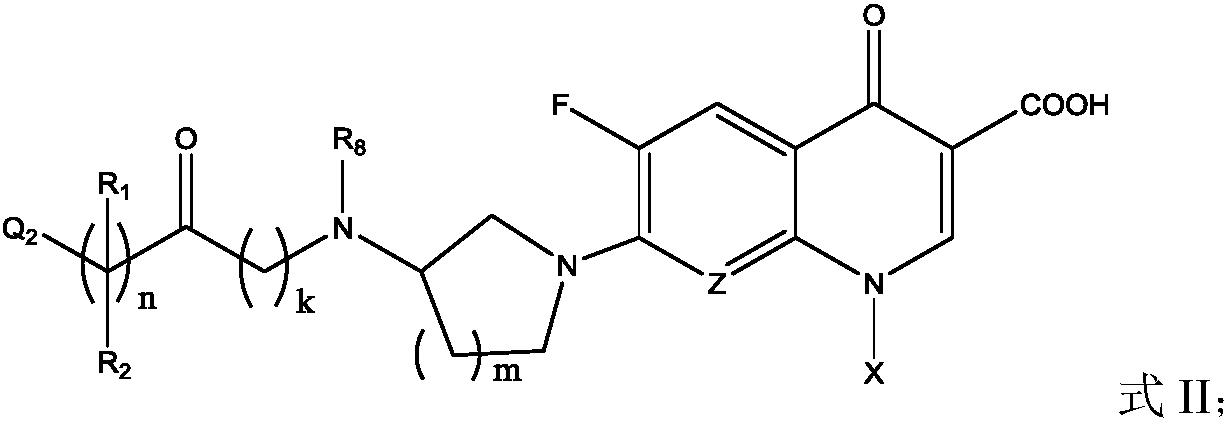
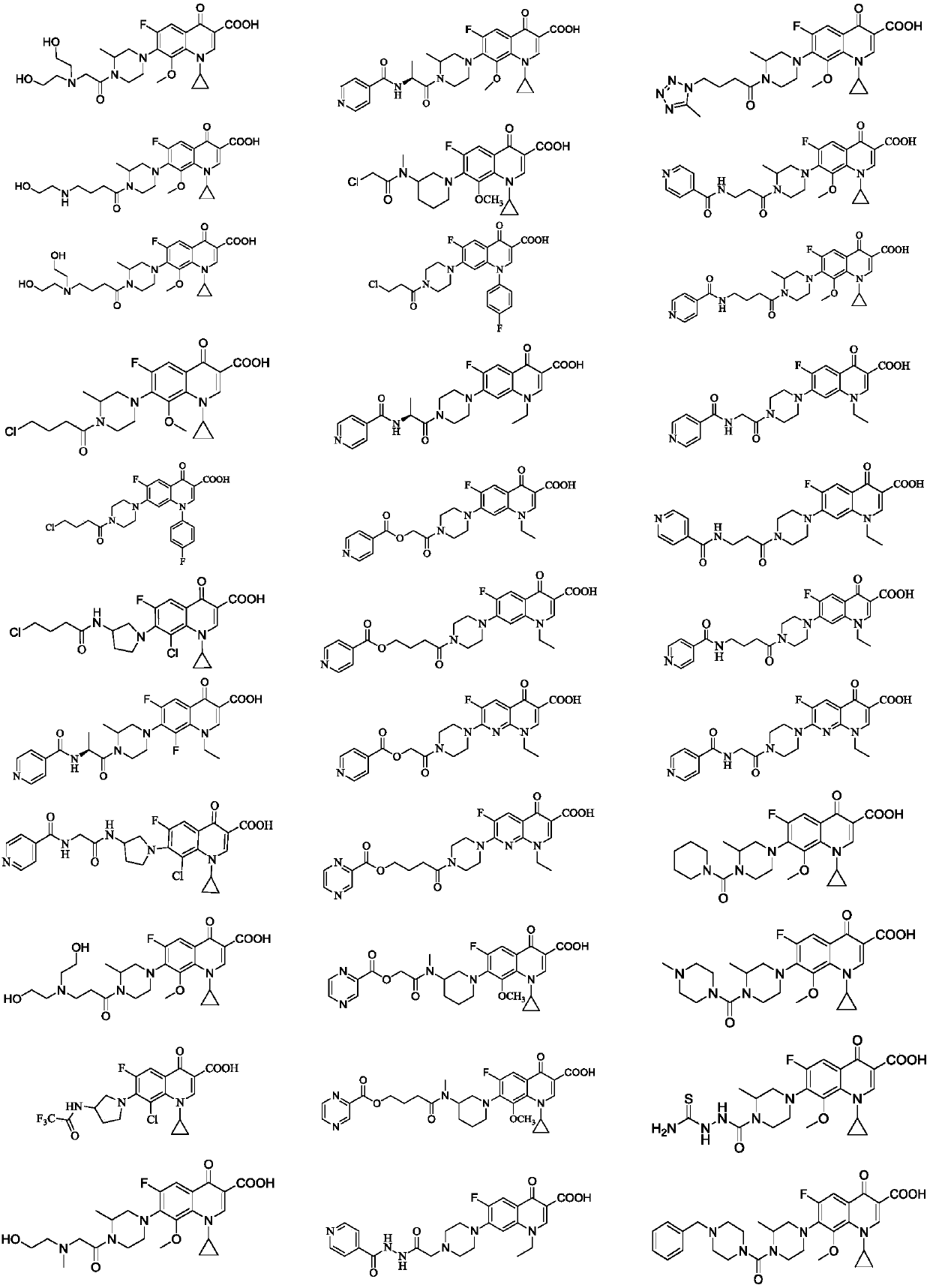
Fluoroquinolone amino derivatives and use thereof in prevention and control of citrus diseases
An amino and alkyl technology, applied in the field of fluoroquinolone amino derivatives, can solve problems such as lack of research and accumulation, lack of technical reserves and systematic research, and no effective and reliable control technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Add raw materials gatifloxacin (GAT) 1mmol, NaHCO 3 2.5mmol, DCM 5mL, add dropwise a mixed solution of chloroacetyl chloride 2mmol and DCM 2mL under ice-cooling. After dropping, the reaction was continuously stirred under ice bath, and the reaction progress was monitored by TLC. After the reaction, add a little ice-cold saturated NaCl solution to dissolve the solid, adjust the pH to 4-5 with ice-cold 2N HCl solution, stir evenly, transfer to a separatory funnel for liquid separation, extract twice with DCM, combine the organic phases, and wash with saturated NaCl solution. Water Na 2 SO 4 Dry and remove the solvent by rotary evaporation. The pure product was obtained by recrystallization or column chromatography, dried and weighed to obtain the product, [M+H] + 453.1342.
Embodiment 2
[0059] Into a 100mL round bottom flask, add raw materials GAT 1mmol, NaHCO 3 2.5mmol, DCM 5mL, add dropwise a mixed solution of chloropropionyl chloride 2mmol and DCM 2mL under ice-cooling. After dropping, the reaction was continuously stirred under ice bath, and the reaction progress was monitored by TLC. After the reaction, add a little ice-cold saturated NaCl solution to dissolve the solid, adjust the pH to 4-5 with ice-cold 2N HCl solution, stir evenly, transfer to a separatory funnel for liquid separation, extract twice with DCM, combine the organic phases, and wash with saturated NaCl solution. Water Na 2 SO 4 Dry and remove the solvent by rotary evaporation. The pure product was obtained by recrystallization or column chromatography, dried and weighed to obtain the product, [M+H] + 467.1453.
Embodiment 3
[0061] Into a 100mL round bottom flask, add raw materials GAT 1mmol, NaHCO 3 2.5mmol, DCM 4mL, add dropwise a mixed solution of 4-chlorobutyryl chloride 2.5mmol and 2mL DCM under ice-cooling. After dropping, the reaction was continuously stirred under ice bath, and the reaction progress was monitored by TLC. After the reaction, add a little ice-cold saturated NaCl solution to dissolve the solid, adjust the pH to 4-5 with ice-cold 2N HCl solution, stir evenly, transfer to a separatory funnel for liquid separation, extract twice with DCM, combine the organic phases, and wash with saturated NaCl solution. Water Na 2 SO 4 Dry and remove the solvent by rotary evaporation. Recrystallized to obtain pure product, dried, weighed to obtain product, [M+H] + 481.1679.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com