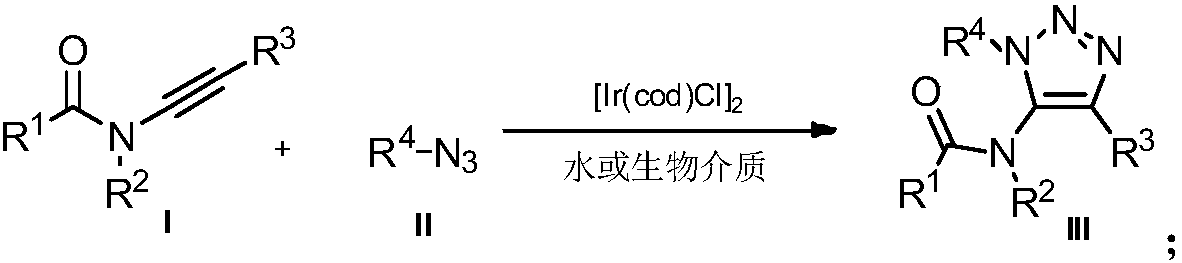
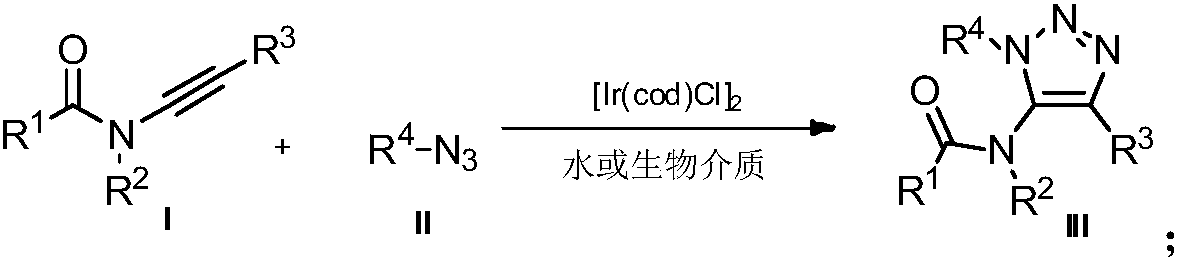
Method for preparing 5-acylamino-1,4,5-trisubstituted 1,2,3-triazole in water phase and biological medium
A biological medium and three-substitution technology, applied in organic chemistry and other fields, to achieve high reaction efficiency and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1: Preparation of 1-benzyl-4-phenyl-5-(pyrazolin 2-one group)-1H-1,2,3-triazole
[0021] Under air, 3-phenylethynyl-pyrazolin 2-one (0.2 mmol, 37.4 mg) was added to water (2 mL), followed by benzyl azide (0.3 mmol, 40.2 mg) and [Ir( COD)Cl] 2 (0.005mmol, 3.3mg), the reaction mixture was stirred at room temperature, and reacted for 12h. After the reaction, it was extracted with ethyl acetate, the solvent was selected to be dry, and the product was separated by column chromatography to obtain 54.4mg of a yellow solid product with a yield of 85%.
Embodiment 2
[0022] Example 2: Preparation of 1-benzyl-4-p-methoxyphenyl-5-(pyrazolin 2-one)-1H-1,2,3-triazole
[0023] 3-p-Methoxyphenylethynyl-pyrazolin 2-one (0.2 mmol, 43.4 mg) was added to water (2 mL) followed by benzyl azide (0.3 mmol, 40.2 mg) under air and [Ir(COD)Cl] 2 (0.005mmol, 3.3mg), the reaction mixture was stirred at room temperature, and reacted for 12h. After the reaction, it was extracted with ethyl acetate, the solvent was selected to be dry, and the product was separated by column chromatography to obtain 60.9mg of a yellow liquid product with a yield of 87%.
Embodiment 3
[0024] Example 3: Preparation of 1-benzyl-4-p-chlorophenyl-5-(pyrazolin 2-one)-1H-1,2,3-triazole
[0025] Under air, 3-p-chlorophenylethynyl-pyrazolin 2-one (0.2 mmol, 44.2 mg) was added to water (2 mL), followed by benzyl azide (0.3 mmol, 40.2 mg) and [ Ir(COD)Cl] 2 (0.005mmol, 3.3mg), the reaction mixture was stirred at room temperature, and reacted for 12h. After the reaction, it was extracted with ethyl acetate, the solvent was selected to be dry, and the white solid product was separated by column chromatography to obtain 56.7mg, with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com