Anti-staphylococcus aureus antibody combination preparation
A staphylococcus, golden yellow technology, applied in the direction of antibodies, antibacterial drugs, antibacterial immunoglobulin, etc., can solve problems such as adverse effects of vitality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[1888] Example 1: Synergistic protective effect of antibody combinations neutralizing recombinant Staphylococcus aureus leukocidin mixture
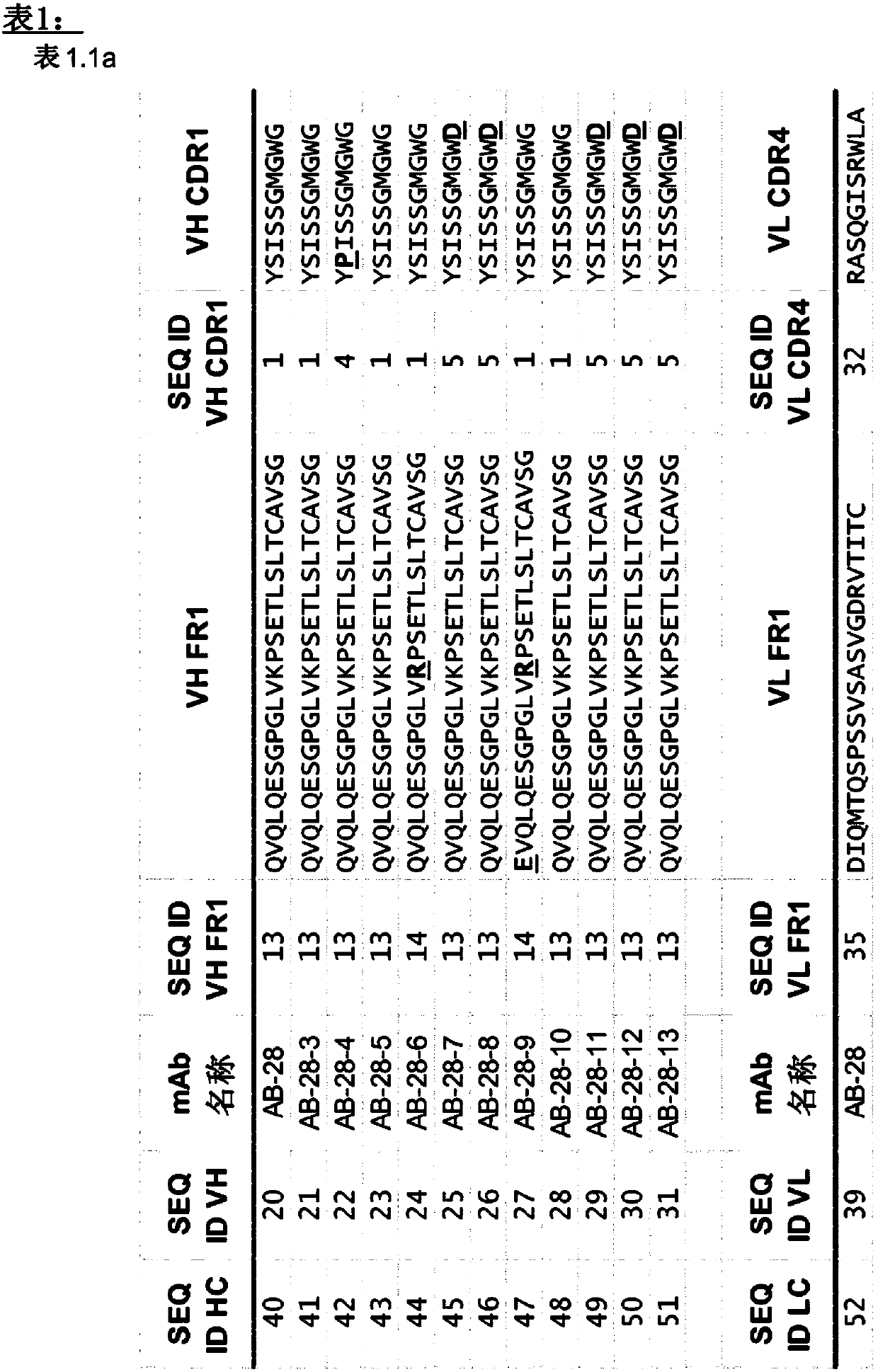
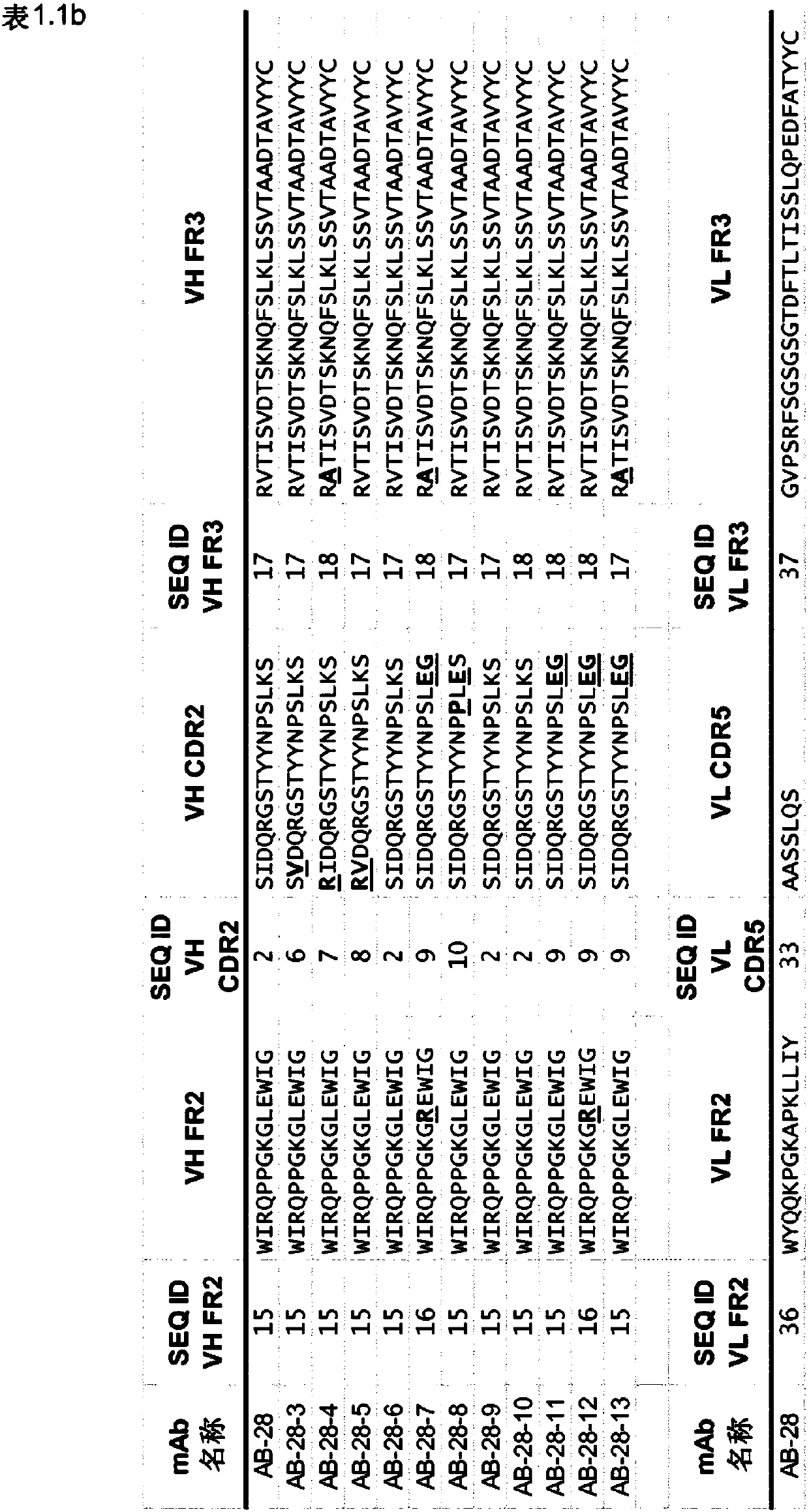
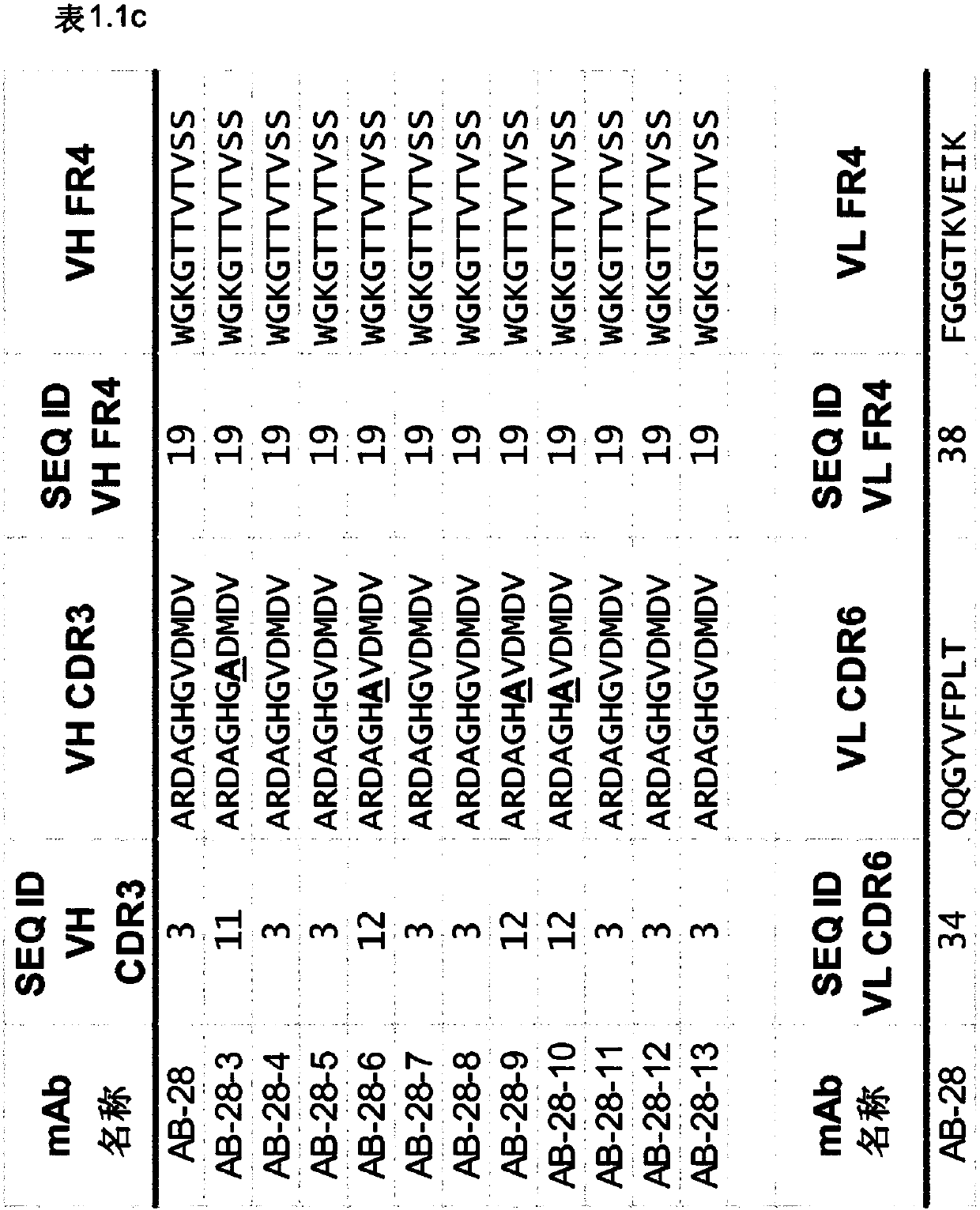
[1889] The synergistic effect of the toxin cross-reactive mAb ASN-1 (Rouha, 2015) was demonstrated using a series of antibodies comprising the CDR sequences of AB-28 or its variant AB-28-x. An antibody comprising the CDR sequences of AB-28 or its variant AB-28-x (eg, an antibody of Table 1) is referred to herein as ASN-1. Such mAbs neutralize alpha-hemolysin, LukSF, LukED, HlgAB and HlgCB.
[1890] ASN-1 mAb was tested alone or with the LukGH neutralizing antibody ASN-2.
[1891] In the present invention, LukGH comprising the CDR sequences of AB-29, AB-30, AB-31, AB-32, AB-33, AB-34, AB-35 and AB-36 or a variant of any of the foregoing Neutralizing antibodies are referred to as ASN-2 mAbs, such as those in Table 2.1, 2.2, 2.3, 2.4, 2.5, 2.6, 2.7 or 2.8.
[1892] Examples employ a series of CDR sequences comprising AB-30, AB-31, AB-32...
example 2
[1997] Example 2: Protection of human PMNs from Staphylococcus aureus culture supernatant mediated by toxin neutralizing antibody combinations to kill
[1998] The number of toxins encoded by genome-different S. aureus strains ranged from three to five, and the lukSF and lukED genes were not present in all strains. S. aureus secretes cytolytic leukocidins into the culture supernatant, and their levels in the supernatant are usually highest during the stationary growth phase.
[1999] According to this example, any mAb with the designation AB-28, AB-28-10, AB-28-7, AB-28-8 or AB-28-9 is compatible with the designation AB-30-3, AB-31, AB - Any mAb combination of 32-9, AB-34-6 or AB-34.
[2000] To test the independent and combined inhibitory ability of antitoxin mAbs on secreted exotoxins, bacterial culture supernatants (CS) were formulated in RPMI supplemented with 1% casamino acids (Amresco). Bacteria were cultured from a single colony to a stationary phase in 20 ml medi...
example 3
[2007] Example 3. Toxin neutralizing antibody combination prevents the toxin produced by live cells of Staphylococcus aureus from killing human PMN
[2008] It has been reported that Staphylococcus aureus can upregulate the expression of leukocidin when it encounters human PMN (Malachowa, 2011). Therefore, it is very interesting to test whether toxin-neutralizing antibodies are able to counteract the effects of bacterial toxins not only after pre-incubation of pre-formed toxins in culture supernatants, but also of bacterial toxins produced in situ in response to human phagocytes. important. For this purpose, PMNs were infected with live bacteria, and the viable cells were detected by flow cytometry to determine their viability.
[2009] Purified PMNs derived from human heparinized whole blood were diluted to 4x10 in RPMI supplemented with 10% FBS and L-glutamine 6 cells / mL. Staphylococcus aureus strains were grown to mid-log phase at 37°C with 200 rpm shaking (NewBrunswic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com