Use of an immunomodulatory composition in the treatment of malignant effusion
A technology of immune regulation and composition, which is applied in the direction of drug combination, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems such as low efficiency, inability to cure the cause, and high requirements
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Embodiment 1 contains the preparation of polyIC composition (BSG) (following stabilizer is an example with kanamycin sulfate)
[0087] PI and PC are mixed according to the ratio between 0.5-1.5, add 200-2000IU kanamycin sulfate and 0.02-10mMCaCl 2 The solution was fixed to a certain volume with PBS, reacted for 30-60 minutes at a temperature not exceeding 60 degrees Celsius, filtered and subpackaged, and the filling volume was about 2ml / cartridge. Wherein the specific preparation examples are as follows.
[0088]
[0089]
Embodiment 2
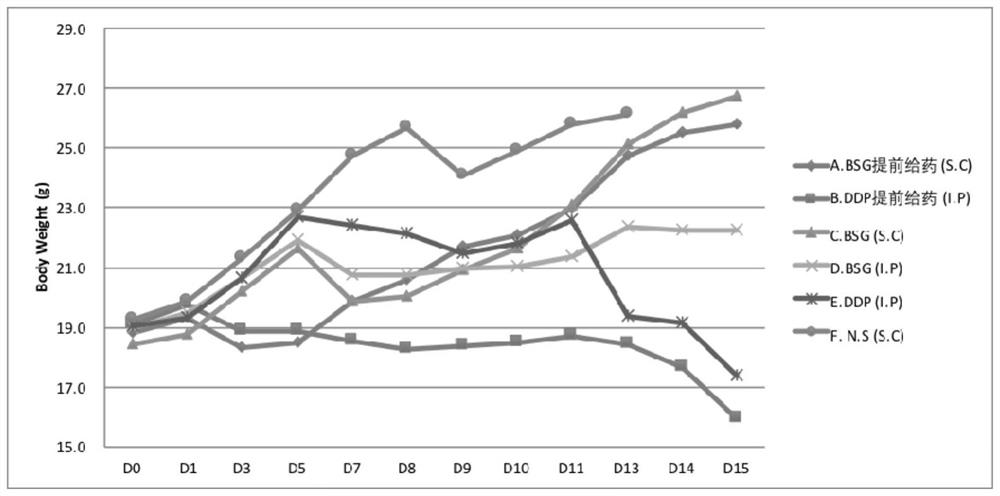
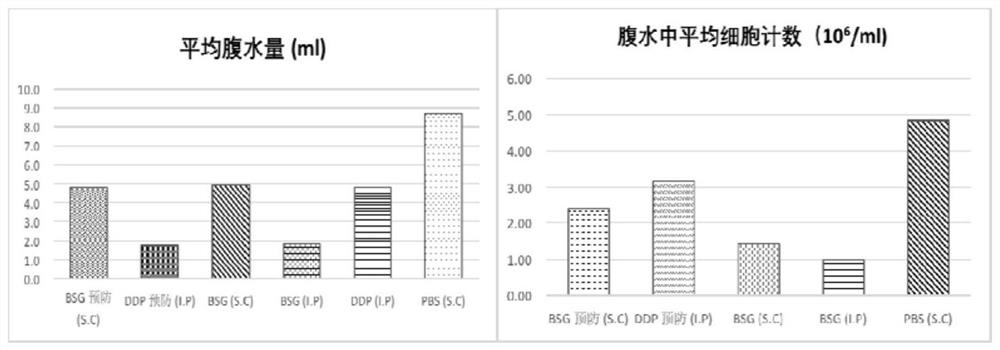
[0090] Example 2 Saizheng (BSG) inhibits and treats ascites in H22 ascites tumor mice
[0091] Experimental background and purpose: Malignant ascites is a fluid in the peritoneal cavity caused by the transfer of digestive system tumors or ovarian cancer through blood vessels and lymphatic vessels, tumor tissue ulceration of the serosa layer, or radical surgery, often due to the shedding of cancer cells and implantation metastasis in the peritoneal cavity. Abnormal accumulation. This experiment was to investigate the inhibitory and therapeutic effects of BSG on ascites in H22 ascites tumor mice.
[0092] Experimental materials: mouse liver cancer cell line H22, female mice, 6-8 weeks old, 18-22g. Saizheng (BSG, containing PIC 2mg / ml, the same below). The control drug cisplatin (DDP), sterile N.S (ie normal saline).
[0093] experimental method:
[0094] 1. Establishment of the H22 ascites tumor model: Take out the frozen ascites cells, thaw them, add them to the medium for ...
Embodiment 3
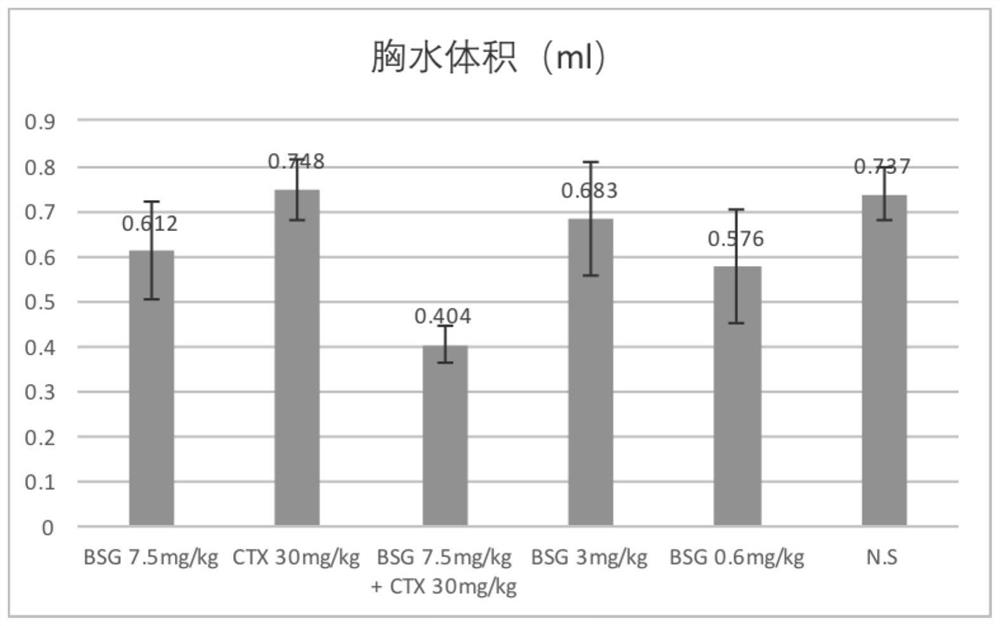
[0117] Embodiment 3 The inhibitory and therapeutic effect of the composition Saizheng (BSG) of the present invention on mouse malignant pleural effusion
[0118] Experimental background and purpose: Malignant pleural effusion usually refers to the pleural effusion caused by the metastasis of malignant tumor in the pleura or other parts to the pleura, accounting for about 50% of all clinical pleural effusions. This experiment explores the inhibitory and therapeutic effects of the composition of the present invention (BSG) on the generation of malignant pleural effusion in mice.
[0119] Experimental materials: mouse liver cancer cell line H22 (purchased from CCTCC), BALB / c mice, SPF grade, female, 6-8 weeks old, 18-22g, purchased from Beijing Weitong Lihua. Saizheng (BSG, 2mg / ml). The control drug Adorson (cyclophosphamide for injection), CTX, lot number 6D111A, 0.2g, Baxter Oncology GmbH. Sodium chloride injection, manufacturer Zhejiang Tianrui Pharmaceutical Co., Ltd., batc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com