Method for preparing tetrasulfur tetranitride by ammoniation of disulfur dichloride
A technology of disulfur dichloride and tetrasulfur nitride, which is applied in the fields of chemical instruments and methods, nitrogen compounds, inorganic chemistry, etc., can solve the problems of complex reaction, low yield and poor safety of the preparation method, and achieve The effect of small equipment investment, less three wastes, and simple process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
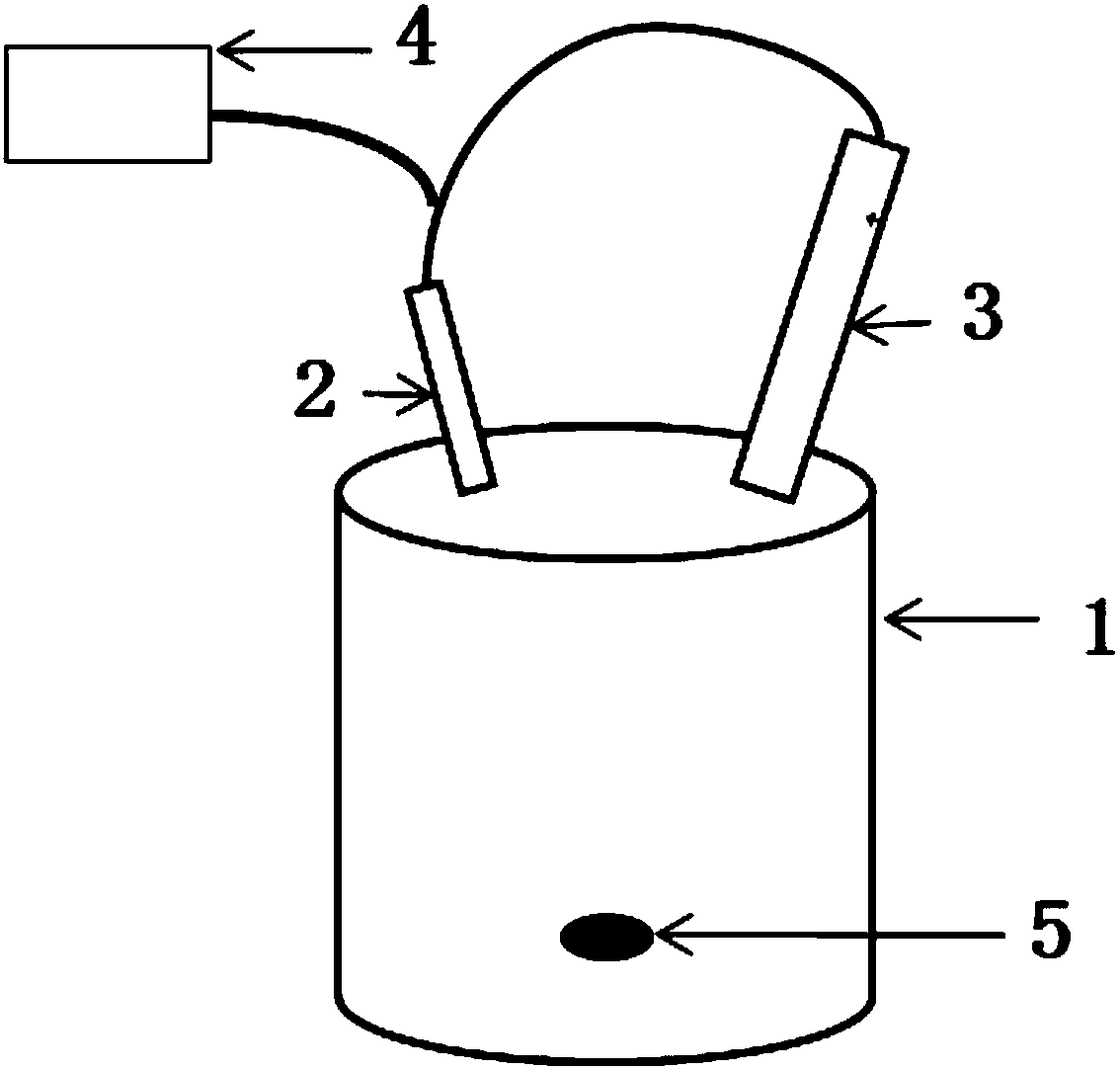
[0025] Take 5ml S 2 Cl 2 In a 250ml two-necked flask, dilute to 100ml with anhydrous ether. In order to reduce solvent loss, place the flask in an ice-water bath, feed ammonia gas into one end, and connect a spherical condenser tube to the other end. The gas outlet of the spherical condenser tube communicates with the ammonia gas inlet. Under strong magnetic stirring, circulate ammonia gas at 1000ml / min for about 35min (if the reaction raw material S 2 Cl 2 Doubling the time for feeding ammonia gas is extended by 1.3 to 1.6 times), and during the reaction, it is observed that the liquid in the reactor changes from light yellow to brown, and obvious solids are produced. Add 10ml of anhydrous diethyl ether every 10 minutes of reaction. When the reactant in the reactor is a completely uniform golden-red liquid-solid mixture, take a part of the mixture and dilute it 5 times with distilled water to test its pH. If pH>8, the reaction can be stopped.
[0026] The liquid-solid mixt...
example 2
[0031] Take 10ml S 2 Cl 2 In a 250ml two-necked flask, dilute to 150ml with anhydrous ether. In order to reduce solvent loss, place the flask in an ice-water bath, feed ammonia gas into one end, and connect a spherical condenser tube to the other end. The gas outlet of the spherical condenser tube communicates with the ammonia gas inlet. Under strong magnetic stirring, ammonia gas was circulated at 1100ml / / min for 1h. During the reaction, it was observed that the liquid in the reactor changed from light yellow to brown, and there were obvious solids that continued to react. Add 10ml of anhydrous diethyl ether every 15 minutes of reaction. When the reactant in the reactor is a completely uniform golden-red liquid-solid mixture, take a part of the mixture and dilute it 5 times with distilled water to test its pH. If pH>8, the reaction can be stopped.
[0032] The liquid-solid mixture obtained in the reaction was separated, and the obtained solid was rinsed with anhydrous ether ...
example 3
[0037] Take 15ml S 2 Cl 2 In a 500ml two-necked flask, dilute to 200ml with anhydrous ether. In order to reduce solvent loss, place the flask in an ice-water bath, feed ammonia gas at one end, and connect a spherical condenser tube at the other end. The gas outlet of the spherical condenser tube communicates with the ammonia gas inlet. Under strong magnetic stirring, ammonia gas was circulated at 1200ml / / min for 1.2h. During the reaction, it was observed that the liquid in the reactor changed from light yellow to brown at the beginning, and there were obvious solids. 20ml of anhydrous diethyl ether. When the reactant in the reactor is a completely uniform golden-red liquid-solid mixture, take part of the mixture and dilute it 5 times with distilled water to test its pH, and the reaction can be stopped when pH>8. According to experience, the reaction is complete in about 1.2h.
[0038] The liquid-solid mixture obtained by the reaction was separated by suction filtration with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com