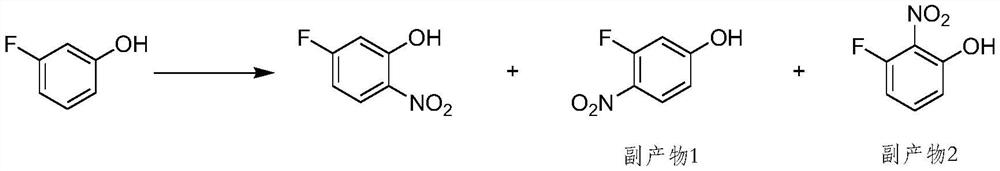
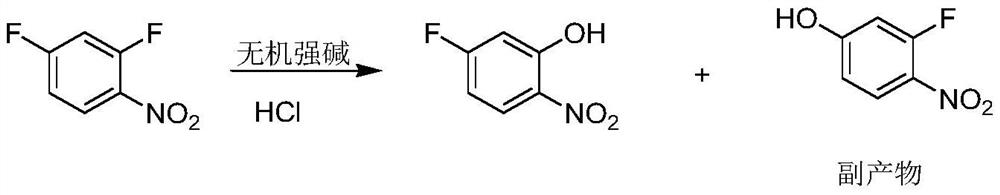
The preparation method of 5-fluoro-2-nitrophenol
A technology of nitrophenol and difluoronitrobenzene is applied in the field of preparation of 5-fluoro-2-nitrophenol, can solve the problems of unfavorable industrial operation, long reaction time, low conversion rate of synthesis method, etc. Short, easy to operate, no side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
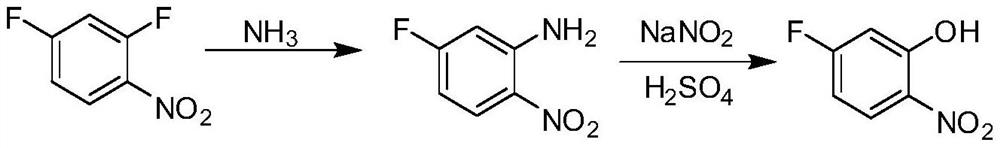
[0025] The preparation method of 5-fluoro-2-nitrophenol comprises the following steps:
[0026] a) 2,4-difluoronitrobenzene and NH 3 Reaction to obtain 5-fluoro-2-nitroaniline;
[0027] b) Dissolving 5-fluoro-2-nitroaniline in sulfuric acid aqueous solution first, then adding sodium nitrite aqueous solution dropwise to the above aqueous solution at 0°C-10°C, and then reacting at 0°C-10°C for 0.5-1h, Then within 1~2h, the temperature was raised to 90~95°C for 1h.
[0028] As a preferred solution of the present invention, in the preparation method of the above-mentioned 5-fluoro-2-nitrophenol, the NH described in step a) 3 For concentrated ammonia.
[0029] In the preparation method of the above-mentioned 5-fluoro-2-nitrophenol, the NH described in step a) 3 The molar ratio to 2,4-difluoronitrobenzene is 2~4:1. Preferably, the NH 3 The molar ratio to 2,4-difluoronitrobenzene is 2.1~2.5︰1.
[0030] In the above-mentioned preparation method of 5-fluoro-2-nitrophenol, the re...
Embodiment 1
[0038] Synthesis of embodiment 1 5-fluoro-2-nitroaniline
[0039] Add 127.5 g of concentrated ammonia water into a 500 mL reaction flask, and add 159 g (1 mol) of 2,4-difluoronitrobenzene at room temperature. Start stirring, and slowly raise the temperature to 40°C for 3 hours. After the raw materials disappeared in the central control analysis, it was cooled to 5-10°C under stirring to crystallize, and 152.9 g of 5-fluoro-2-nitroaniline was obtained by filtration, with a yield of 98%.
Embodiment 2
[0040] Example 2 Synthesis of 5-fluoro-2-nitroaniline
[0041] Add 180g of water into a 500mL reaction flask, and add 159g (1mol) of 2,4-difluoronitrobenzene at room temperature. Turn on the stirring, feed 42.5 g of ammonia gas at 35-40°C, and then keep it warm for 3 hours. After the raw materials disappeared in the central control analysis, it was cooled to 5-10°C under stirring to crystallize, and 153.2 g of 5-fluoro-2-nitroaniline was obtained by filtration, with a yield of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com