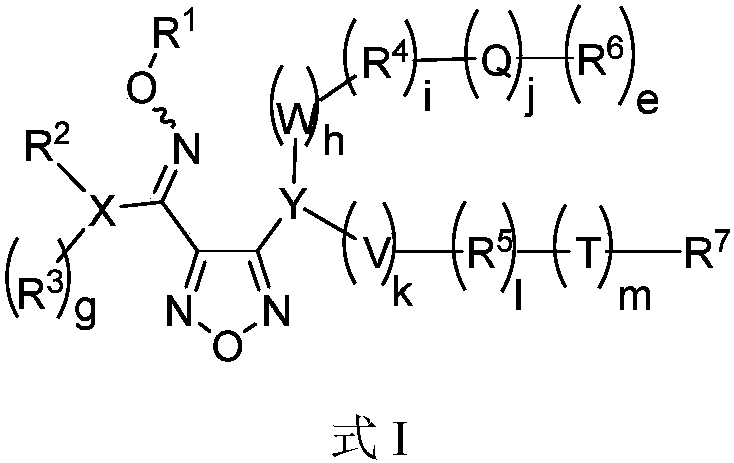
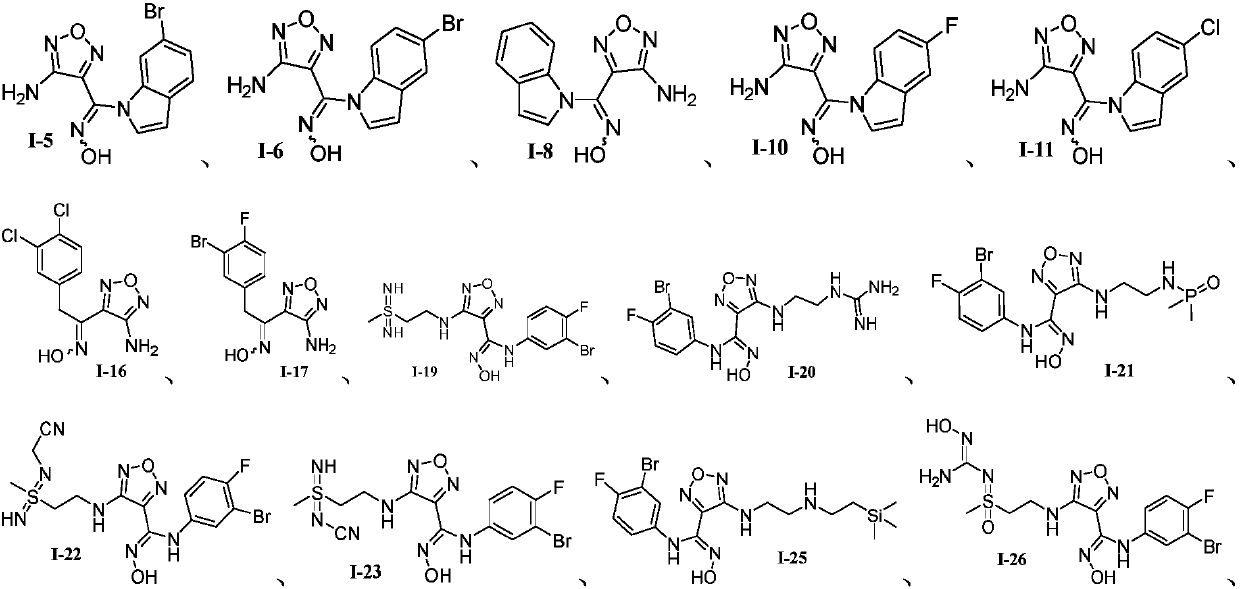
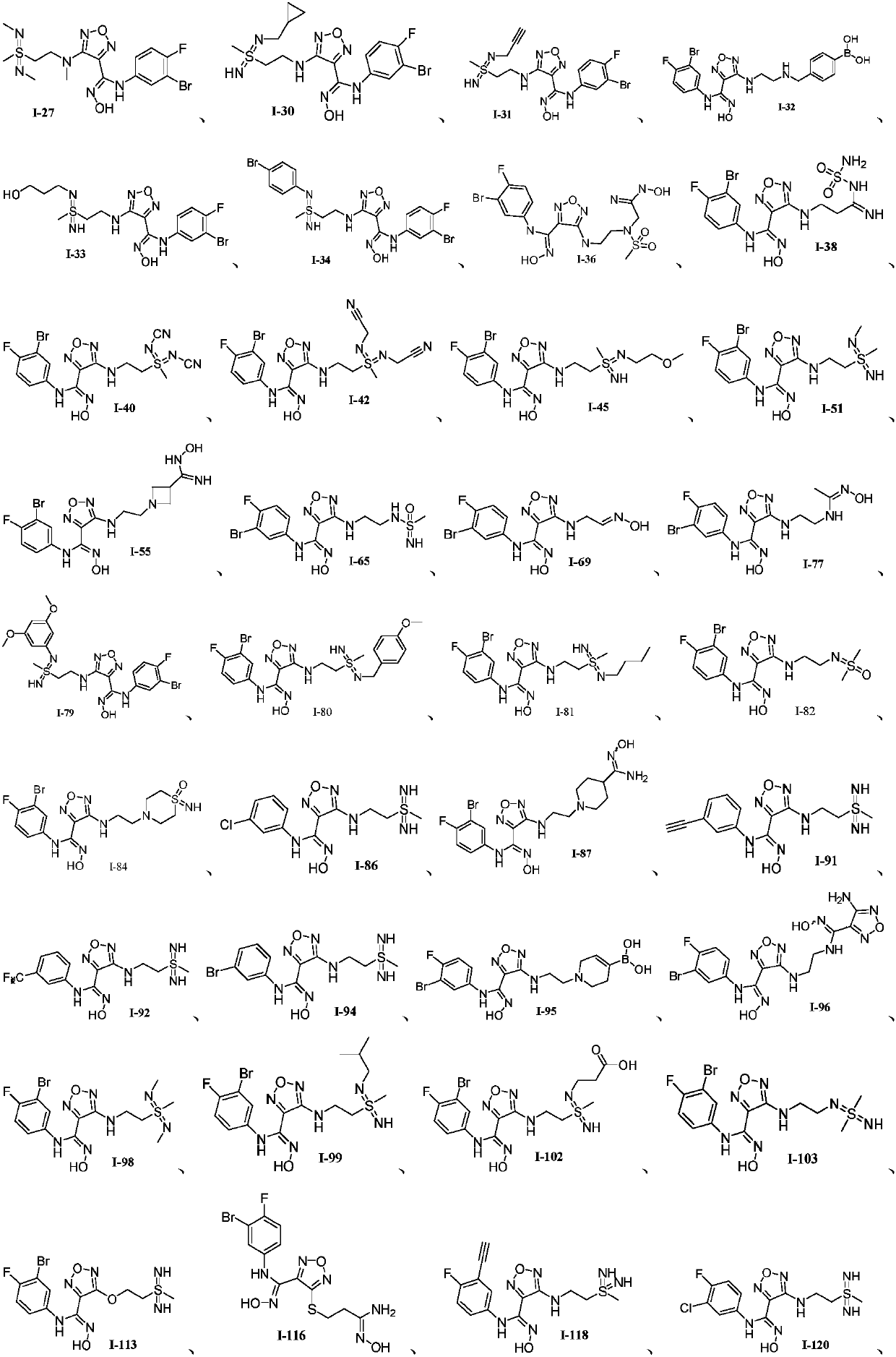
Oxadiazole-contained cyclic compound, preparation method, intermediates, compositions and application thereof
A technology containing an oxadiazole ring and a compound is applied in the fields of drug combination, compounds containing elements of Group 3/13 of the periodic table, chemical instruments and methods, and can solve the problems of IDO inhibitor activity and unsatisfactory pharmacokinetic properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0555]
[0556] first step:
[0557] Malononitrile (compound shown in formula 1-a) was dissolved in water (280mL), heated to 45°C, added sodium nitrite (15.2g, 220mmol), and stirred until the solution was clear. Cool the solution to 0°C, add concentrated hydrochloric acid (13.2mL), stir in the ice bath for 5 minutes, remove the ice bath and stir for 1.5 hours, add 50% aqueous hydroxylamine solution (19.8g, 599 mmol) at 0°C, and warm to room temperature And stirred for 1 hour, heated to reflux and stirred for 24 hours. The reaction solution was cooled to room temperature, and a white solid was precipitated, which was filtered to obtain the compound 4-aminoethyl-N'-hydroxyl-1,2,5-oxadiazole-3-carboxamidine (25g) shown in 1-b, a white solid . 1 H NMR (400MHz, DMSO) δ 10.46 (s, 1H), 6.27 (s, 2H), 6.18 (s, 2H). LC-MS: m / z: (M+H)+=144.0.
[0558] Step two:
[0559] 4-aminoethyl-N'-hydroxyl-1,2,5-oxadiazole-3-carboxamidine (25g, 174.7mmol) (compound as shown in formula 1-b) is...
preparation example 2
[0569]
[0570] first step:
[0571]The compound (2.6g, 16mmol) shown as formula 1-c was dissolved in dry dichloromethane (40mL), under ice-cooling, 2-methylthio-1-ethylamine (1.6g, 17.6mmol) was added In the reaction solution, after stirring for ten minutes, triethylamine (2.424 g, 24 mmol) was added and reacted for 1 hour. After the reaction was completed, dichloromethane was distilled off under reduced pressure, ethyl acetate was added, the organic phase was washed once with water, once with saturated sodium chloride solution, dried over anhydrous sodium sulfate, and the organic phase was evaporated to dryness under reduced pressure to obtain the formula 2-a The compound 4-amino-N'-hydroxy-N-(2-methylthioethyl)-1,2,5-oxadiazole-3-carboxamidine (3.128g) was yellow oil. 1H NMR(400MHz,CD3Cl)δ6.75(s,1H),5.74(s,1H),5.16(s,2H),3.84(q,2H),2.72(t,2H),2.12(s,3H) .
[0572] Step two:
[0573] The compound represented by Formula 2-a (1 g, 4.6 mmol) was added to 15 mL of water, ...
preparation example 3
[0584]
[0585] first step:
[0586] The method is the same as that described in the first step of Preparation Example 2. Using 1-c as the starting substrate, the compound 4-amino-N'-hydroxyl-N-(2-methoxyethyl)-1,2,5-oxadiazole- 3-Formamidine (6.6g), yellow oil. 1H NMR (400MHz, DMSO) δ10.67(s,1H),6.28(s,2H),6.14(t,J=6.4Hz,1H),3.54(dd,J=12.0,5.9Hz,2H),3.38 (t, J=5.7Hz, 2H), 3.22(s, 3H).LC-MS: m / z: (M+H)+=202.0.
[0587] Step two:
[0588] The method is described in the second step of Preparation Example 2. Using 3-a as the starting material, the compound N'-hydroxyl-4-((2-methoxyethyl)amino)-1,2,5-oxadiazole-3- Formamidine (6.06g), yellow oil. 1H NMR (400 MHz, DMSO) δ10.54(s, 1H), 6.23(s, 2H), 6.16(t, J=5.8Hz, 1H), 3.52(t, J=5.3Hz, 2H), 3.39( dd, J=11.0, 5.4Hz, 2H), 3.29(s, 3H). LC-MS: m / z: (M+H)+=202.0.
[0589] third step:
[0590] The method is described in the third step of Preparation Example 2. Using 3-b as the starting material, the compound N-hydroxyl-4-((2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com