Application of sophocarpidine to preparation of medicine for inhibiting neuroinflammation
A technology of neuroinflammation and matrine, which is applied in the application field of matrine in the preparation of anti-neurinflammation drugs, can solve the problems of complex pathogenesis, special site, and lack of therapeutic drugs in ND, and achieve high clinical application value and development Prospect, effect of inhibiting neuroinflammatory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
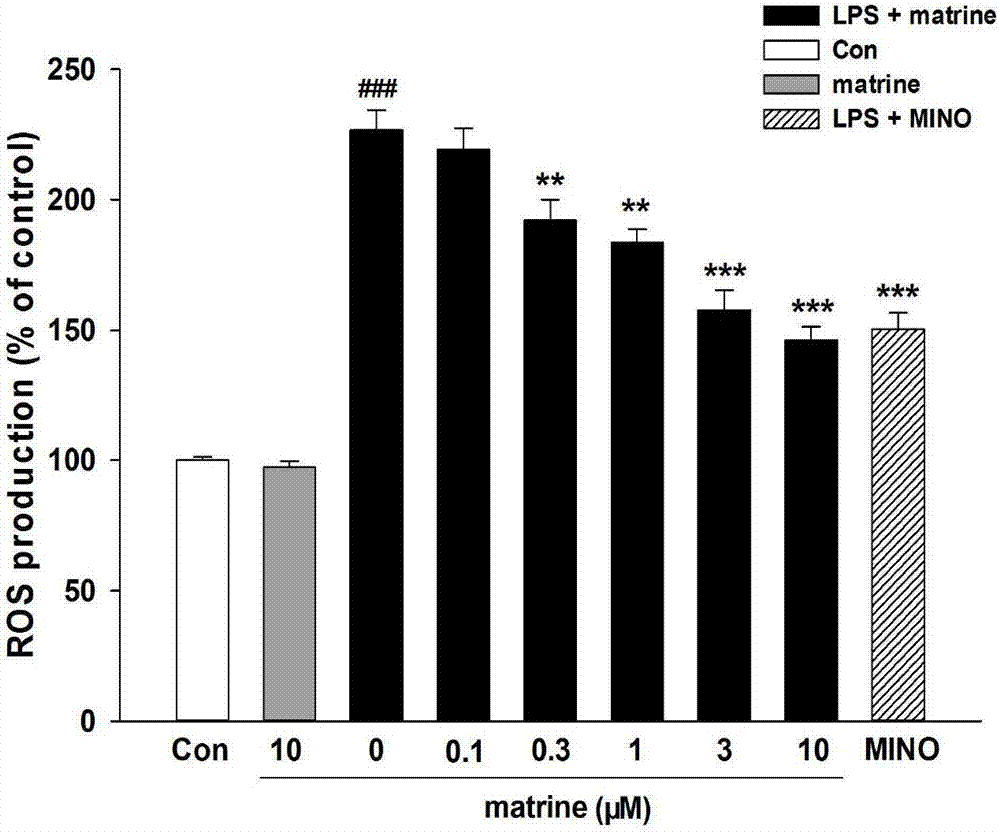
[0029] DCFH oxidation method was used to investigate the effect of matrine on the release of reactive oxygen species (ROS) from microglial cells induced by LPS;
[0030] Cell line: mouse microglial cell line BV-2;
[0031] Drugs: LPS; matrine; MINO.
[0032] Experimental principle: DCFH-DA (2′,7′-dichlorodihydrofluororescein diacetate) can enter the cell and undergo a deacetylation reaction to generate DCFH. DCFH is a non-fluorescent substance, which can be oxidized by intracellular ROS to generate fluorescent substance DCF, and the amount of ROS release can be reflected by detecting the fluorescence intensity. The specific operation steps are as follows: DCFH-DA was dissolved in pure methanol to prepare a mother solution with a concentration of 10 mM, and the mother solution was diluted 500 times with Hank's balanced salt solution (HBSS) to a final concentration of 20 μM before use. Take the BV-2 cells after drug treatment, suck the supernatant, incubate with the 20 μM DCFH...
Embodiment 2
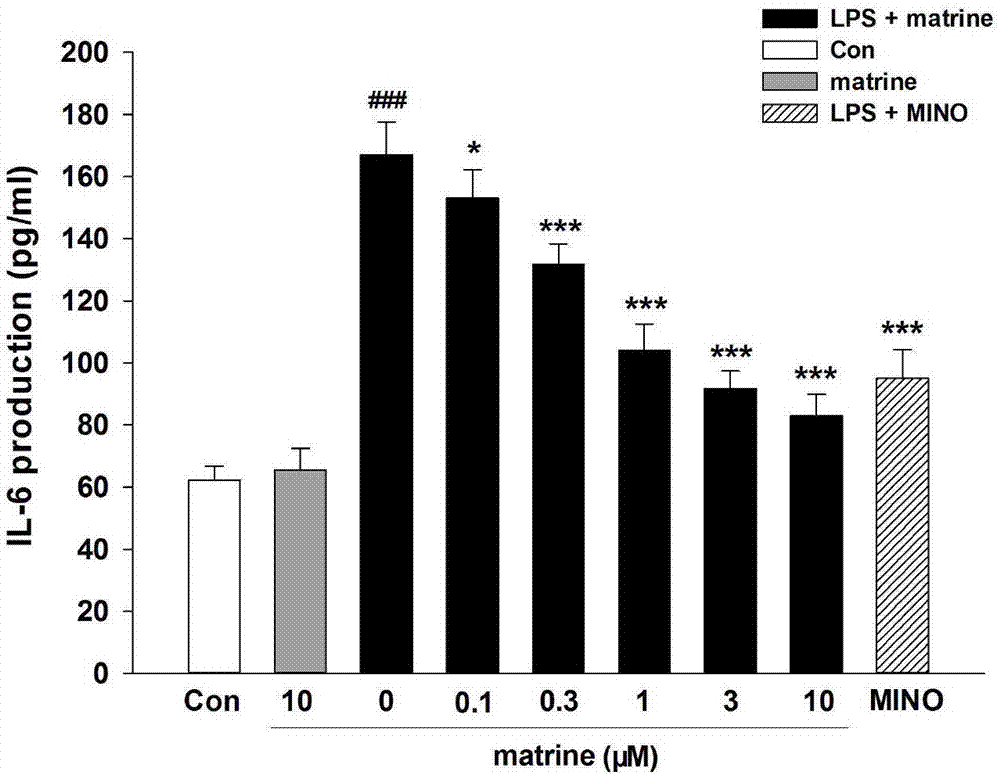
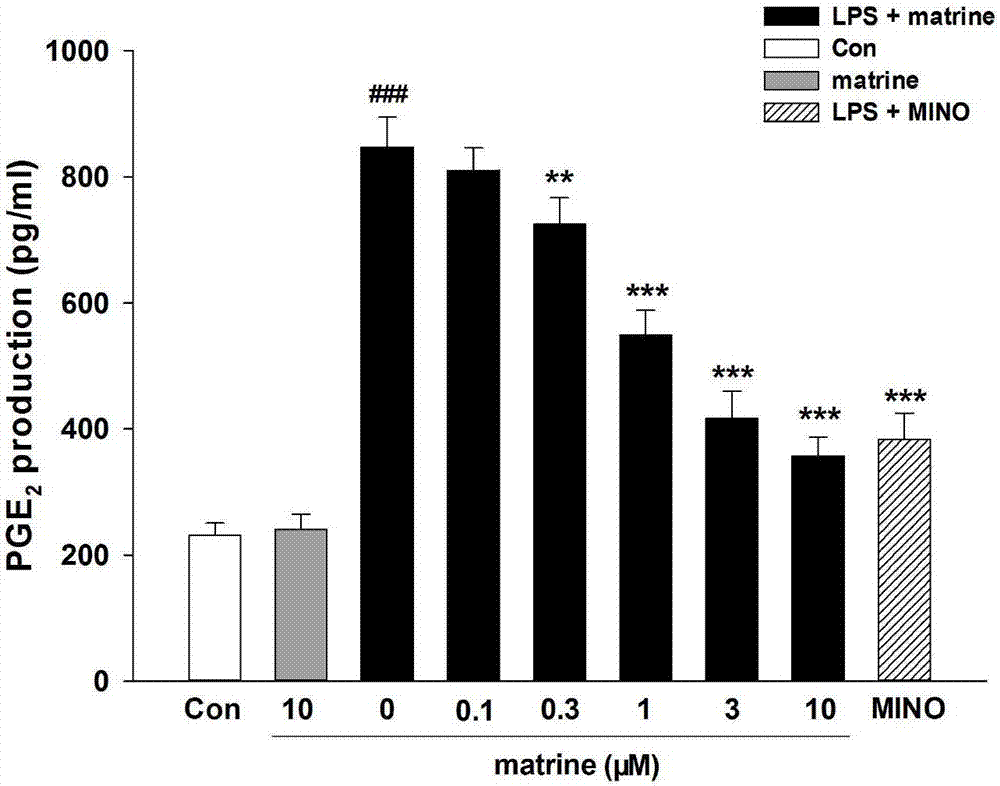
[0036] Effect of matrine on LPS-induced microglial interleukin-6 (IL-6) and prostaglandin E by ELISA 2 (PGE 2 ) release effects;
[0037] Cell line: mouse microglial cell line BV-2;
[0038] Drugs: LPS; matrine; MINO.
[0039] Detection method: Take BV-2 cells in the logarithmic growth phase, inoculate the cells in a 6-well plate with fresh serum-free DMEM medium, and the cell density is 3×10 5 cells / ml, inoculum volume 600μl / well, placed in an incubator at 37°C, 5% CO 2 cultivated under conditions. After the cells adhered to the wall for 3 hours, the culture solution was carefully sucked out, and according to the experimental group, the cells were replaced with different drugs prepared in serum-free DMEM culture solution to incubate the cells. At the same time, a blank control group was set up, and each group was set with 3 replicate wells, and the drug addition to the cells continued. After culturing for 2-4 hours, collect the supernatant, and detect IL-6 and PGE in the...
Embodiment 3
[0043] Iba-1 immunohistochemical staining to investigate the effect of matrine on the activation of microglial cells in the mouse brain induced by LPS;
[0044] Animals: C57BL / 6 mice;
[0045] Drugs: matrine, donepezil, LPS;
[0046] Experimental method: 72 C57BL / 6 mice were randomly divided into 6 groups: normal control group, model control (Aβ) group, matrine high, medium and low dose groups (40, 20, 10mg / kg) and positive drug Donepezil hydrochloride 5mg / kg group. Animals in each group were lightly anesthetized with chloral hydrate and fixed their heads. The skin on the surface of the skull was disinfected with 75% ethanol. The lateral ventricle was positioned 1 mm to the right of the midpoint of the line connecting the two eyes and 2 mm behind the line connecting the posterior corners of the eyes. 2 mm into a sterile micro-syringe, and then slowly inject LPS solution (1 mg / ml, 3 μl / rat), and the normal control group is injected with an equal volume of normal saline. From...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com