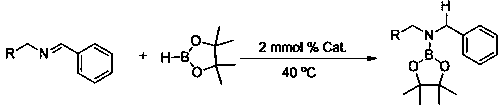
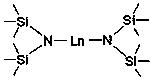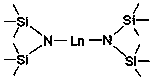Application of Disilazide Rare Earth Complex in Catalytic Hydroboration of Imine and Borane
A rare earth complex, catalytic imine technology, applied in catalytic reactions, organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, etc. Good yield and other problems, to achieve the effects of easy product post-processing, fast reaction speed, and reduced catalyst dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment one: Yb[N(SiMe 3 ) 2 ] 2 Catalytic synthesis of amino borates from benzylidene aniline and pinacol borane
[0025] Under an inert gas atmosphere, add benzylidene aniline (90.62 mg, 0.5 mmol) to the reaction flask after dehydration and deoxygenation treatment, and then add Yb[N(SiMe 3 ) 2 ] 2 Acetonitrile solution (0.1 mL, 0.01mmol), then add pinacol borane (63.99mg, 0.5mmol) with a pipette gun, react at 40°C for 3h, then add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 88%. NMR data of the product: 1 H NMR (400 MHz, CDCl3) δ 7.33 – 7.17 (m, 10H, ArH), 4.73 (s, 2H, NCH 2 ), 1.33 (s, 12H, CH 3 ).
Embodiment 2
[0026] Embodiment two: Eu[N(SiMe 3 ) 2 ] 2 Catalytic synthesis of amino borates from benzylidene aniline and pinacol borane
[0027] Under an inert gas atmosphere, add benzylidene aniline (90.62 mg, 0.5 mmol) to the reaction flask after dehydration and deoxygenation treatment, and then add Eu[N(SiMe 3 ) 2 ] 2 Acetonitrile solution (0.1 mL, 0.01mmol), then add pinacol borane (63.99mg, 0.5mmol) with a pipette gun, react at 40°C for 3h, then add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 82%. NMR data of the product: 1 H NMR (400 MHz, CDCl3) δ 7.33 – 7.17 (m, 10H, ArH), 4.73 (s, 2H, NCH 2 ), 1.33 (s, 12H, CH 3 ).
Embodiment 3
[0028] Embodiment three: Sm[N(SiMe 3 ) 2 ] 2 Catalytic synthesis of amino borates from benzylidene aniline and pinacol borane
[0029] Under an inert gas atmosphere, add benzylidene aniline (90.62 mg, 0.5 mmol) to the reaction flask after dehydration and deoxygenation treatment, and then add Eu[N(SiMe 3 ) 2 ] 2 Acetonitrile solution (0.1 mL, 0.01mmol), then add pinacol borane (63.99mg, 0.5mmol) with a pipette gun, react at 40°C for 3h, then add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 80%. NMR data of the product: 1 H NMR (400 MHz, CDCl3) δ 7.33 – 7.17 (m, 10H, ArH), 4.73 (s, 2H, NCH 2 ), 1.33 (s, 12H, CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com