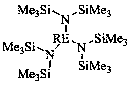Application of trisilylamine rare earth complex in preparation of borate ester by catalyzing reaction of ester and borane
A technology of rare earth complexes and trisilicon amines, applied in organic compound/hydride/coordination complex catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problem of high catalyst dosage and unstable chemical properties of catalysts and other problems, to achieve the effects of easy product post-treatment, simple and controllable reaction process, and wide application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment one: La[N(SiMe 3 ) 2 ] 3 Catalytic synthesis of boronate from benzyl benzoate and pinacol borane
[0021] Under nitrogen atmosphere, the catalyst La[N(SiMe 3 ) 2 ] 3 (3.1mg, 0.005mmol), add benzyl benzoate (189.8 μL, 1 mmol) with a pipette gun, and then add pinacol borane (319.2 μL, 2.2 mmol) with a pipette gun, react at room temperature for 30min , contact air to terminate the reaction, use a dropper to draw a drop into the NMR tube, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 99%. NMR data of the product: 1 H NMR (400MHz, CDCl 3 ) δ 7.35 – 7.27 (m, 10H, ArH), 4.92 (s, 4H, OCH 2 ), 1.25 (s, 26H,CH 3 ).
Embodiment 2
[0022] Embodiment two: Yb[N(SiMe 3 ) 2 ] 3 Catalytic synthesis of boronate from benzyl benzoate and pinacol borane
[0023] Under nitrogen atmosphere, the catalyst Yb[N(SiMe 3 ) 2 ] 3 (3.3mg, 0.005mmol), add benzyl benzoate (189.8 μL, 1mmol) with a pipette gun, and then add pinacol borane (319.2 μL, 2.2 mmol) with a pipette gun, react at room temperature for 30min, Expose to air to terminate the reaction, use a dropper to draw a drop into the NMR tube, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 10%.
Embodiment 3
[0024] Embodiment three: Y[N(SiMe 3 ) 2 ] 3 Catalytic synthesis of boronate from benzyl benzoate and pinacol borane
[0025] Under nitrogen atmosphere, add catalyst Y[N(SiMe 3 ) 2 ] 3 (2.9mg, 0.005mmol), add benzyl benzoate (189.8 μL, 1mmol) with a pipette gun, and then add pinacol borane (319.2 μL, 2.2 mmol) with a pipette gun, react at room temperature for 30min, Expose to air to terminate the reaction, use a dropper to draw a drop into the NMR tube, add CDCl 3 Dubbed into a solution. Calculated 1 H spectrum yield was 88%. The NMR data of the product are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com