Small molecular polypeptide promoting skin damage repair and application thereof
A technology for small molecular peptides and skin damage, applied in the field of biomedicine, can solve problems such as abnormal skin healing results and incomplete skin healing mechanisms, and achieve the effects of high affinity, reduced degradation and low production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1. Detection of affinity between remodeling peptide SNFLHLG and FGFR2 by ITC method
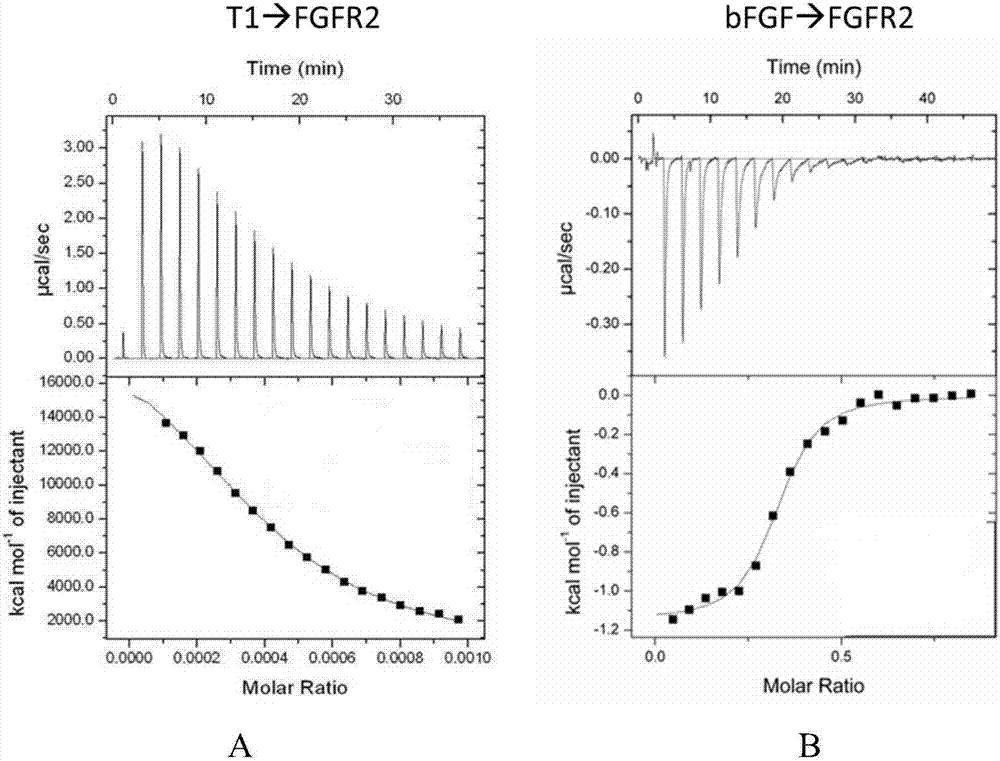
[0032] The affinity detected by isothermal microcalorimeter (ITC) is as follows: figure 1 As shown, Figure A is the detection result of the binding force between the remodeling peptide T1 peptide and FGFR2, and their affinity constant K=3.11E7±1.63E6M -1 , the dissociation constant Kd≈32.1nM, Figure B shows the detection results of the binding force between bFGF and FGFR2, and their affinity constant is K=1.62E5±2.50E4M -1 , the dissociation constant Kd≈6.17 μM. The results showed that the affinity of the remodeling peptide T1 peptide to FGFR2 was much higher than that of bFGF to FGFR2, the former being about 200 times that of the latter. (K represents the affinity constant, Kd represents the dissociation constant, the larger the K, the smaller the Kd, the stronger the affinity).
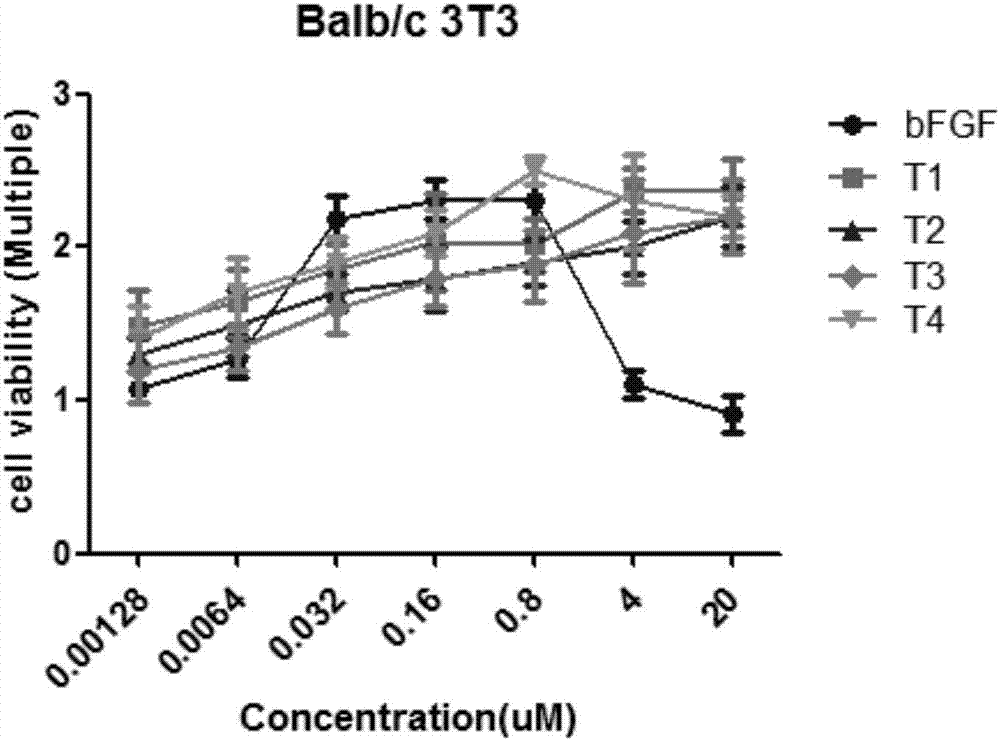
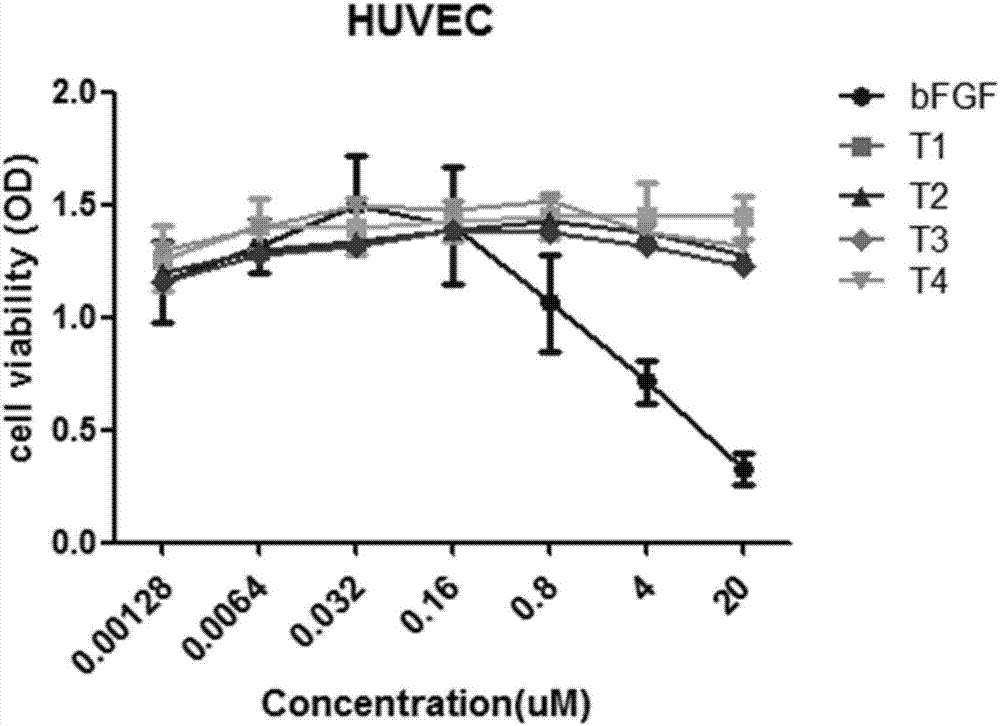
[0033] 2. CCK-8 method was used to detect the pro-proliferative effects of remodeling peptides T1, T2,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com