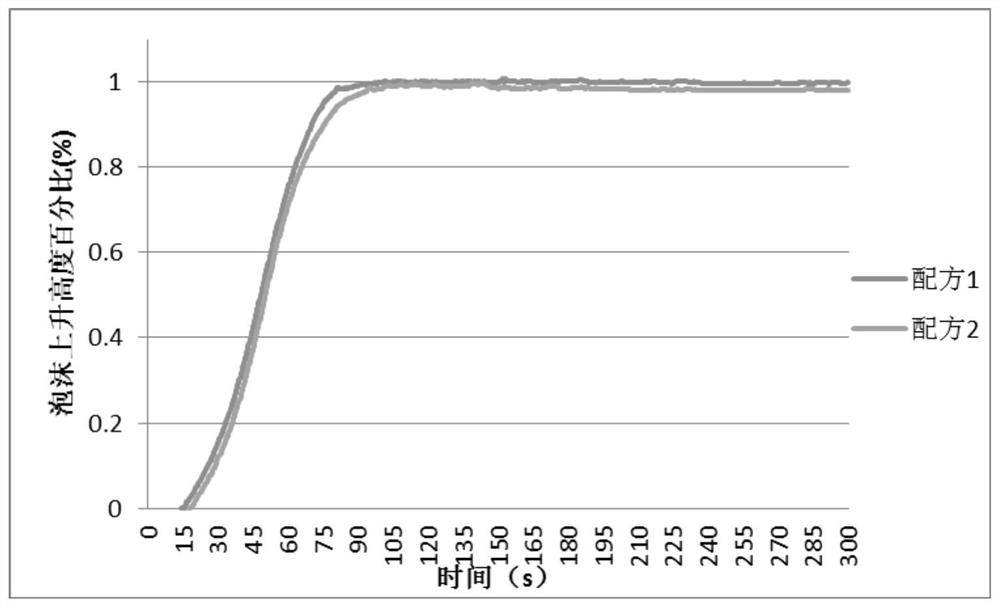
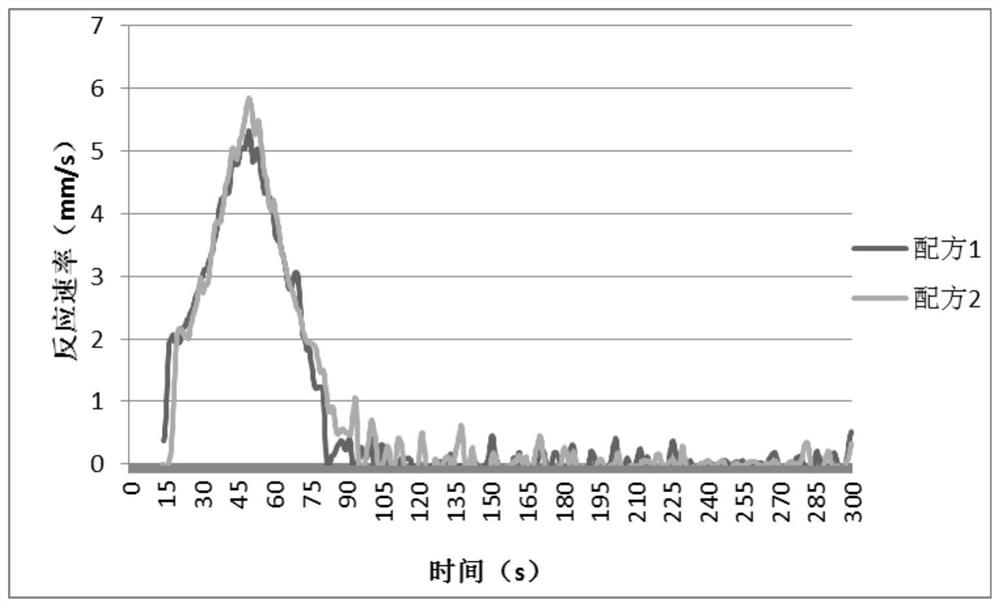
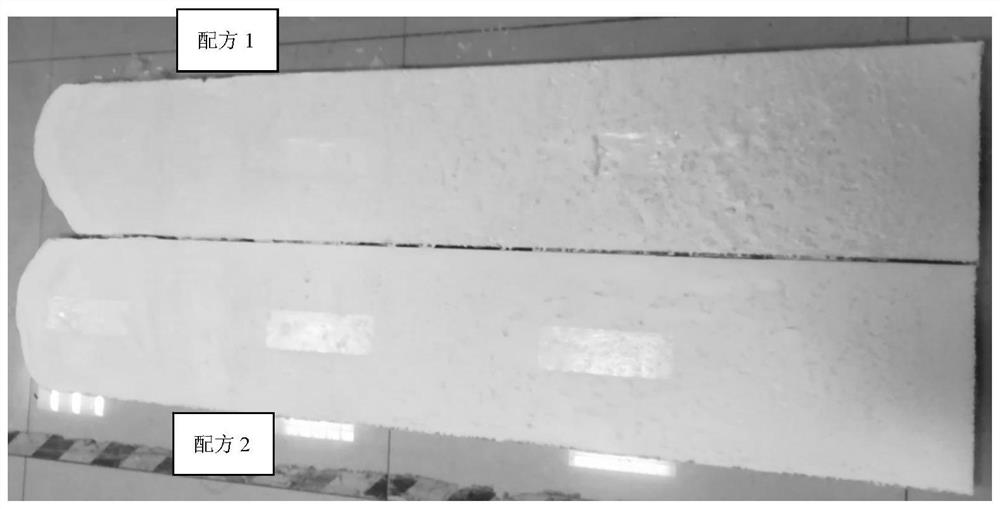
n,n-Dimethyl-4-cyclohexylaminocyclohexylmethane and its preparation method and application
A technology of cyclohexylaminobenzenemethane and cyclohexylmethane, which is applied in the field of by-product ammonia removal mixture, can solve the problems of high cost and low catalytic efficiency of amine catalysts, and achieve reduced production costs, low toxicity, and low amine odor Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of palladium series catalyst 1:
[0044] Dissolve 12.52g of palladium nitrate dihydrate, 2.81g of rhodium nitrate, and 1.38g of ruthenium acetate in 100ml of deionized water, heat to 80°C to form a homogeneous solution, then add 93.5g of activated carbon (average particle size 20μm, specific surface area 220m 2 / g, pore volume 0.35cc / g), in a water bath at 80°C for 4 hours and then gradually evaporated to dryness, then baked in an oven at 120°C for 12 hours; finally moved to a muffle furnace, in an air atmosphere at 2°C / The temperature was raised to 500°C for 6 hours, and the catalyst was obtained after natural cooling. The composition of the catalyst is as follows: Pd is 5wt%, Rh is 1wt%, Ru is 0.5wt%, and the rest is activated carbon, based on the corresponding metal elements accounting for the total mass of the catalyst.
Embodiment 2
[0046] Preparation of Palladium Series Catalyst 2:
[0047] Dissolve 25.04g of palladium nitrate dihydrate, 0.14g of rhodium nitrate, and 2.76g of ruthenium acetate in 100ml of deionized water, heat to 60°C to form a homogeneous solution, then add 88.95g of alumina (average particle size 50μm, specific surface area 180m 2 / g, pore volume 0.30cc / g), in a water bath at 70°C for 5 hours and then gradually evaporated to dryness, then baked in an oven at 100°C for 16 hours; finally moved to a muffle furnace, in an air atmosphere at 3°C / The temperature was raised to 550°C for 8 hours, and the catalyst was obtained after natural cooling. The composition of the catalyst is as follows: 10wt% of Pd, 0.05wt% of Rh, 1wt% of Ru, and the rest are aluminum oxide, based on the weight of corresponding metal elements in the total mass of the catalyst.
Embodiment 3
[0049] Preparation of palladium series catalyst 3:
[0050] Dissolve 5.01g of palladium nitrate dihydrate, 1.40g of rhodium nitrate, and 5.51g of ruthenium acetate in 100ml of deionized water, heat to 70°C to form a homogeneous solution, then add 95.5g of silicon dioxide (average particle size 60μm, specific surface area 240m 2 / g, pore volume 0.38cc / g), in a water bath at 60°C for 6 hours and then gradually evaporated to dryness, then baked in an oven at 120°C for 12 hours; finally moved to a muffle furnace, in an air atmosphere at 2°C / The temperature was raised to 600°C for 6 hours, and the catalyst was obtained after natural cooling. The composition of the catalyst is as follows: Pd is 2wt%, Rh is 0.5wt%, Ru is 2wt%, and the rest is silicon dioxide, based on the corresponding metal elements accounting for the total mass of the catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com