Liraglutide nasal drops for the treatment of type 2 diabetes
A technology of liraglutide and nasal drops, which is applied in the field of nasal drops for the treatment of type II diabetes, can solve the problems of poor patient compliance, low absorption and utilization, and increased drug costs, and achieve the effect of convenient use and reduced pain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
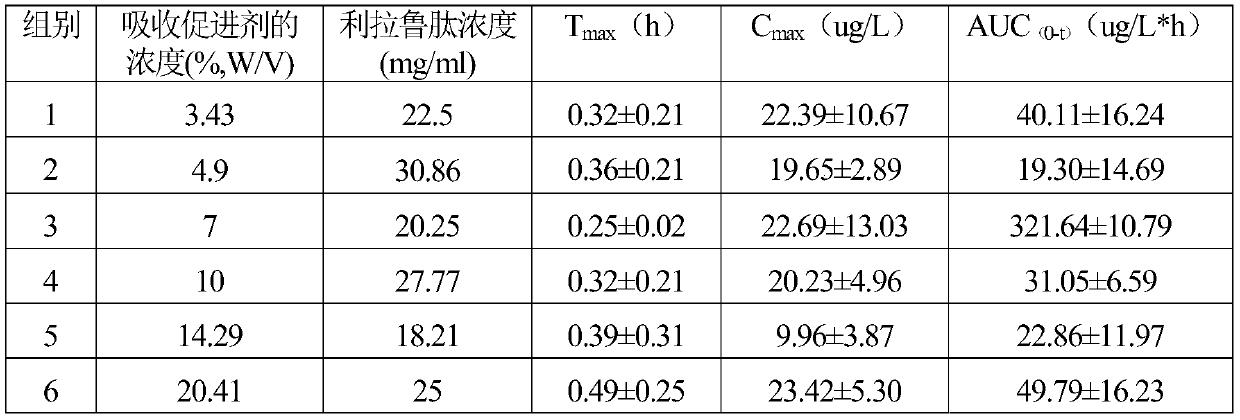
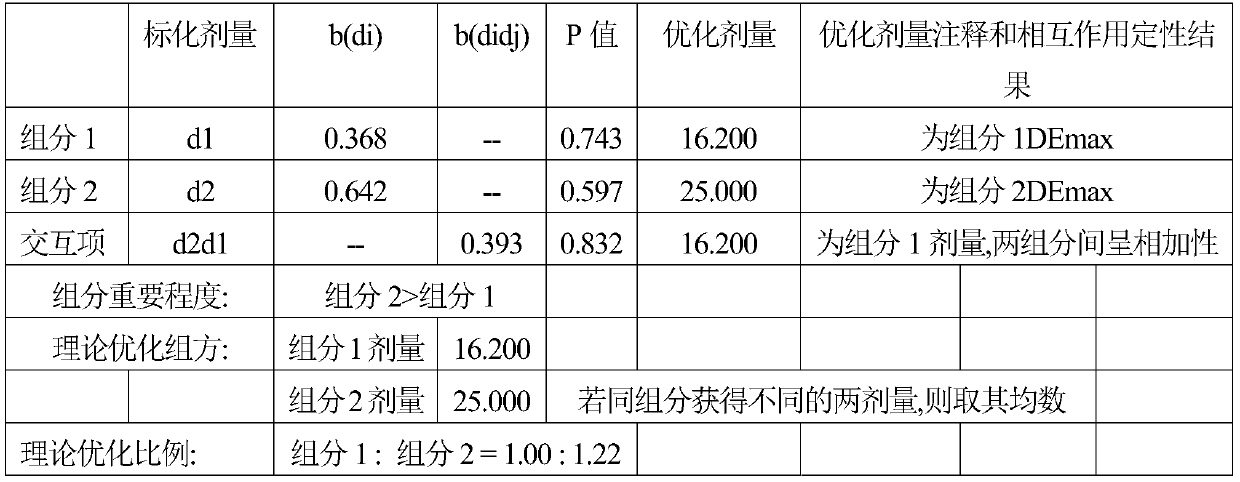
[0024] Taking the preparation of liraglutide nasal drops 1000mL for the treatment of type II diabetes as an example, the following mass ratio raw materials are used to make nasal drops according to the conventional preparation method:
[0025] Liraglutide 14.4g
[0026] Solutol HS15 200g
[0027] Add distilled water to 1000mL
[0028] Its preparation method is as follows:
[0029] Put Solutol HS15 into a container and add 400mL of distilled water, heat to 45°C to dissolve, so that the concentration of Solutol HS15 is 0.3g / mL, store in a refrigerator at 4°C, take it out, add liraglutide, add distilled water to 1000mL, stir, and make It is fully dissolved, bottled, 1.5mL per bottle, sterilized by circulating steam at 100°C for 30 minutes, and made into liraglutide nasal drops, each ml contains 14.4mg of liraglutide, three times a day, nasal drip after meals, Each nasal drop 0.5mL, long-term use.
Embodiment 2
[0031] Taking the preparation of liraglutide nasal drops 1000mL for the treatment of type II diabetes as an example, the following mass ratio raw materials are used to make nasal drops according to the conventional preparation method:
[0032] Liraglutide 14.4g
[0033] Solutol HS15 50g
[0034] Add distilled water to 1000mL
[0035] The preparation method is the same as that of Example 1, and Liraglutide nasal drops are prepared, containing 14.4 mg of liraglutide per milliliter, and the dosage is the same as that of Example 1.
Embodiment 3
[0037] Taking the preparation of liraglutide nasal drops 1000mL for the treatment of type II diabetes as an example, the following mass ratio raw materials are used to make nasal drops according to the conventional preparation method:
[0038] Liraglutide 14.4g
[0039] Solutol HS15 250g
[0040] Add distilled water to 1000mL
[0041] The preparation method is the same as that of Example 1, and Liraglutide nasal drops are prepared, containing 14.4 mg of liraglutide per milliliter, and the dosage is the same as that of Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com