A kind of clopidogrel bisulfate tablet and preparation method thereof
A clopidogrel bisulfate tablet, a technology of clopidogrel bisulfate, which is applied in the field of biomedicine and can solve problems such as incomplete blockage of coronary artery thrombosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
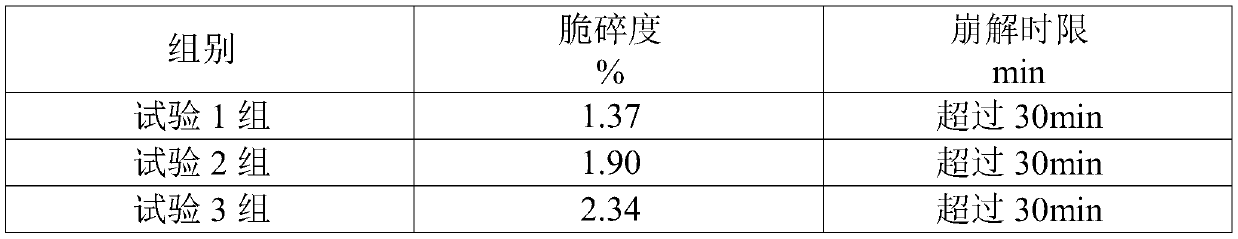
[0077] Clopidogrel hydrogen sulfate 979g, polyethylene glycol 4000 80g, hydrogenated castor oil 33g, methacrylic acid-methyl acrylate copolymer 400g, disintegrant low-substituted hydroxypropyl cellulose 120g, filler microcrystalline cellulose 150g and Mannitol 1100g.
[0078] (1) Clopidogrel hydrogen sulfate was passed through a 40-mesh sieve; mannitol and polyethylene glycol 4000 were passed through a 100-mesh sieve; microcrystalline cellulose, low-substituted hydroxypropyl cellulose, and methacrylic acid-methyl acrylate copolymer were passed through 60 mesh sieve; the above-mentioned raw and auxiliary materials are evenly mixed to obtain spare raw and auxiliary materials;
[0079] (2) Add the spare raw and auxiliary materials to the dry granulator for granulation. The granulation parameters are selected as the feeding speed of 12-13 r / min, the extrusion pressure of 14-18kg, and the extrusion speed of 10-11 r / min. Select a 20-mesh screen, and re-granulate the fine powder wit...
Embodiment 2
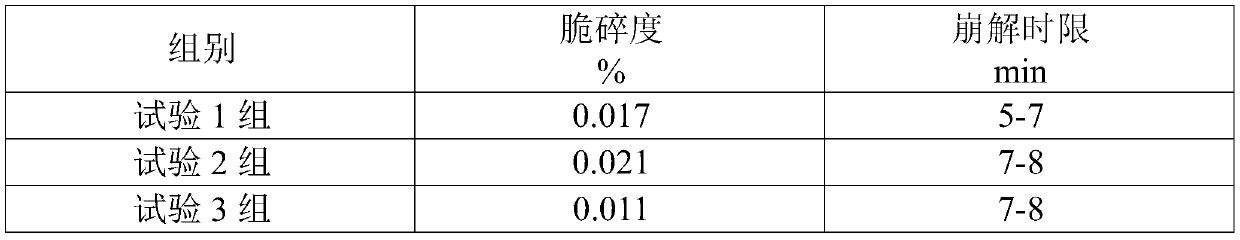
[0081] Clopidogrel hydrogen sulfate 195.8g, polyethylene glycol 4000 16g, hydrogenated castor oil 6.6g, methacrylic acid-methyl acrylate copolymer 80g, disintegrant low-substituted hydroxypropyl cellulose 24g, filler microcrystalline cellulose 30g and mannitol 220g.
[0082] (1) Clopidogrel hydrogen sulfate was passed through a 40-mesh sieve; mannitol and polyethylene glycol 4000 were passed through a 100-mesh sieve; microcrystalline cellulose, low-substituted hydroxypropyl cellulose, and methacrylic acid-methyl acrylate copolymer were passed through 60 mesh sieve; the above-mentioned raw and auxiliary materials are evenly mixed to obtain spare raw and auxiliary materials;
[0083] (2) Add the spare raw and auxiliary materials to the dry granulator for granulation. The granulation parameters are selected as the feeding speed of 12-13 r / min, the extrusion pressure of 14-18kg, and the extrusion speed of 10-11 r / min. Select a 20-mesh screen, and re-granulate the fine powder with...
Embodiment 3
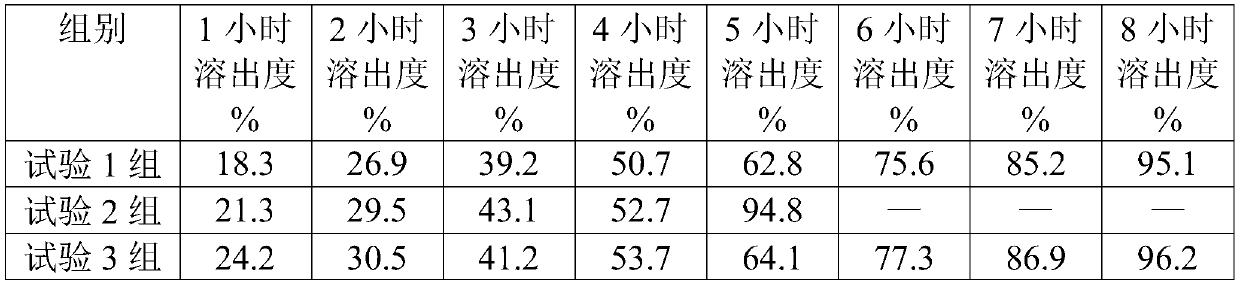
[0085] Clopidogrel hydrogen sulfate 489.5g, polyethylene glycol 4000 40g, hydrogenated castor oil 16.5g, methacrylic acid-methyl acrylate copolymer 200g, disintegrant low-substituted hydroxypropyl cellulose 60g, filler microcrystalline cellulose 75g and mannitol 550g.
[0086] (1) Clopidogrel hydrogen sulfate was passed through a 40-mesh sieve; mannitol and polyethylene glycol 4000 were passed through a 100-mesh sieve; microcrystalline cellulose, low-substituted hydroxypropyl cellulose, and methacrylic acid-methyl acrylate copolymer were passed through 60 mesh sieve; the above-mentioned raw and auxiliary materials are evenly mixed to obtain spare raw and auxiliary materials;
[0087] (2) Add the spare raw and auxiliary materials to the dry granulator for granulation. The granulation parameters are selected as the feeding speed of 12-13 r / min, the extrusion pressure of 14-18kg, and the extrusion speed of 10-11 r / min. Select a 20-mesh screen, and re-granulate the fine powder wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com