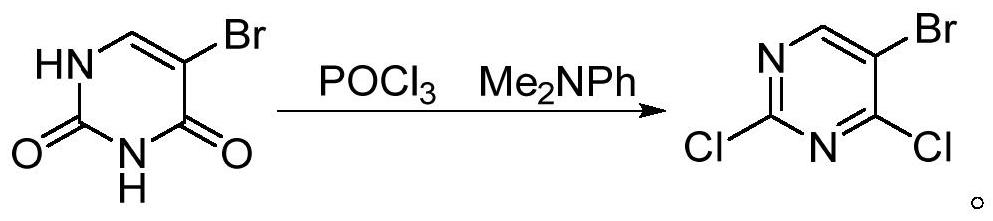Preparation method of halogenated uracil compounds
A compound, uracil technology, applied in the field of preparation of halogenated uracil compounds, can solve the problems of high toxicity of chlorinating reagents or solvents, high reaction temperature, low yield and the like, and achieves easy judgment and control, reflux reaction temperature Low, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 5-bromouracil (6.0g, 31.4mmol) and PCl 5 (16.4 g, 78.5 mmol) were mixed in a reaction flask, 1,2-dichloroethane (50 mL) was added, and the reaction mixture was heated to reflux. During the reaction, the mixture changed from a suspension state to a light yellow clear solution, at this time, TLC showed that the reaction of the raw materials was complete, and the mixture was cooled to room temperature. The reaction mixture was slowly poured into stirred ice water, stirred for 1 h, added DCM (3×50 mL) for extraction, the organic layer was added anhydrous MgSO 4 After drying, the solvent was evaporated to obtain a light yellow transparent liquid. The silica gel column was further purified to obtain a colorless transparent liquid, that is, compound 2 (yield 99%, purity 97.5%).
Embodiment 2
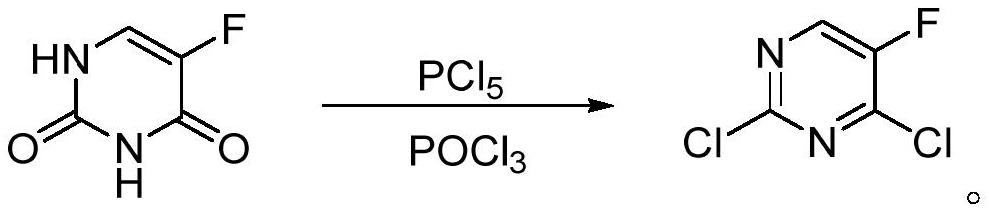
[0041] 5-bromouracil (6.0g, 31.4mmol) and PCl 5 (19.68g, 94.2mmol) mixed in the reaction flask, add SOCl 2 (50 mL), the reaction mixture was heated to reflux. During the reaction, the mixture changed from a suspension state to a light yellow clear solution, at this time, TLC showed that the reaction of the raw materials was complete, and the mixture was cooled to room temperature. The reaction mixture was slowly poured into stirred ice water, stirred for 1 h, added DCM (3×50 mL) for extraction, the organic layer was added anhydrous MgSO 4 After drying, the solvent was evaporated to obtain a light yellow transparent liquid. The silica gel column was further purified to obtain a colorless transparent liquid, that is, compound 2 (yield 99%, purity 97.2%).
Embodiment 3
[0043] 5-bromouracil (6.0g, 31.4mmol) and PCl 5(16.4 g, 78.5 mmol) were mixed in a reaction flask, carbon tetrachloride (50 mL) was added, and the reaction mixture was heated to reflux. During the reaction, the mixture changed from a suspension state to a light yellow clear solution, at this time, TLC showed that the reaction of the raw materials was complete, and the mixture was cooled to room temperature. The reaction mixture was slowly poured into stirred ice water, stirred for 1 h, added DCM (3×50 mL) for extraction, the organic layer was added anhydrous MgSO 4 After drying, the solvent was evaporated to obtain a light yellow transparent liquid. The silica gel column was further purified to obtain a colorless transparent liquid, that is, compound 2 (yield 99%, purity 97%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com