Biomarker for epilepsy diagnosis
A biomarker, epilepsy technology, applied in biological testing, disease diagnosis, biomaterial analysis, etc., can solve the method of shortening the period of antiepileptic drug taking, without serum or cerebrospinal fluid prediction, diagnosis, treatment and prognosis evaluation kit products And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
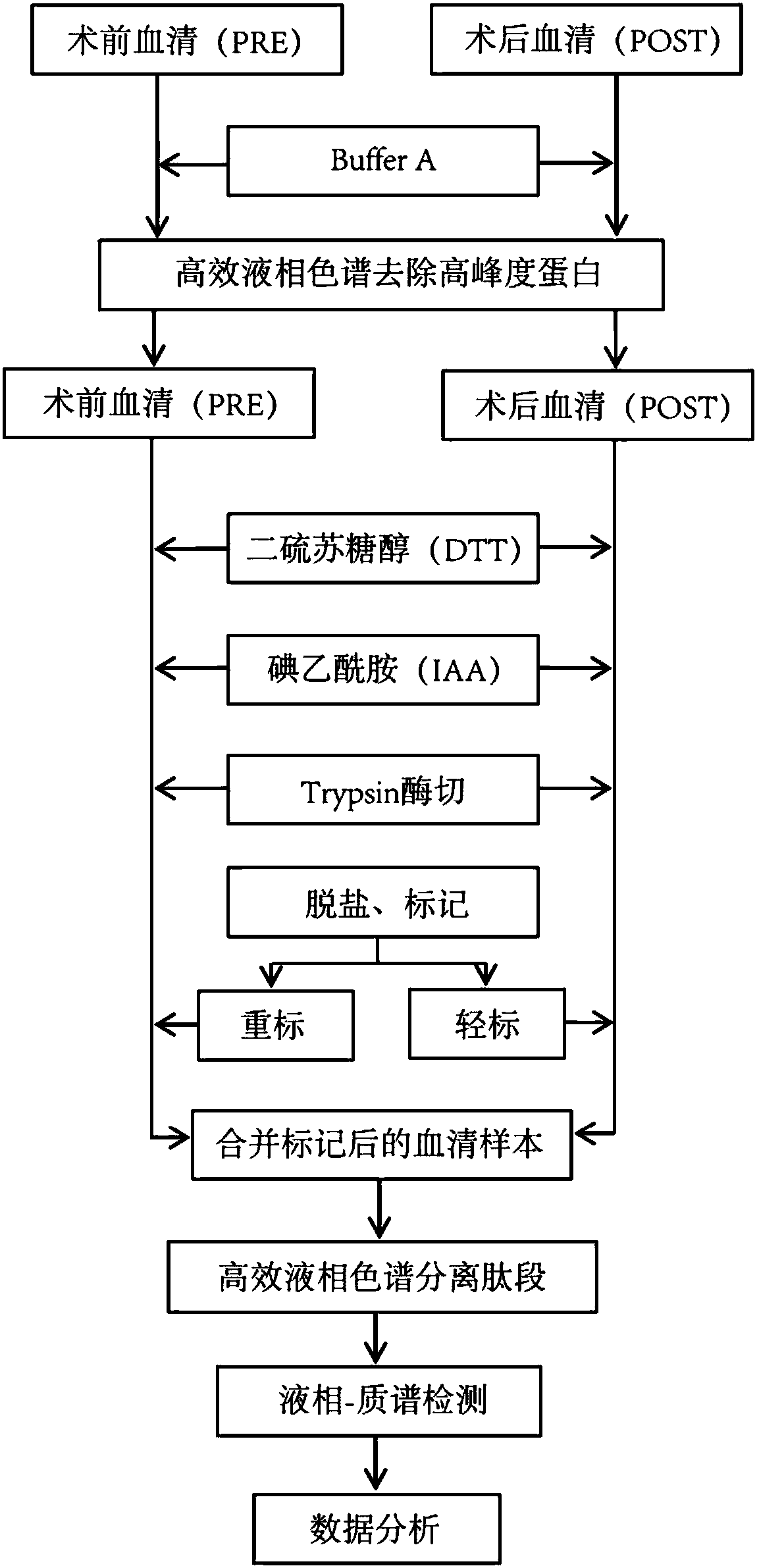
[0031] 1. Sample collection
[0032] There were 6 patients clinically diagnosed as drug-refractory epilepsy (Table 1), all of whom were temporal lobe epilepsy. They all underwent the same surgical treatment: anterior temporal lobe plus hippocampal amygdala resection. Follow-up was carried out after operation. During the follow-up, the 6 patients were all in line with the standard of clinical cure through the verification of EEG and clinical seizures. The peripheral venous blood or arterial blood of the patients was collected according to different time points, the time points were: preoperative, postoperative follow-up for half a year, and postoperative follow-up for one year. 5ml of peripheral blood was drawn each time, centrifuged at 4000rpm / min for 20 minutes, and then the supernatant (serum) was collected and stored at -80°C.
[0033] Table 1. Basic information of clinical samples
[0034]
[0035]
[0036] Case I is the screening experimental sample, and cases II-...
Embodiment approach 2
[0078] Verification experiment based on the above results to expand the sample
[0079] The experimental technical process is the same as the screening experimental technical process ( figure 1 )
[0080] The case numbers of the verification experimental samples are II-VI, and they are cured cases of temporal lobe epilepsy like the screening experimental case I.
[0081] The reagents, proportioning ratio, reaction time, light-heavy labeling and other methods used in the verification experiment are the same as those in the screening experiment.
[0082] Table 3. Sample grouping and labeling methods for verification experiments
[0083] Labeled sample number
Remark (H)
Light (L)
2
II-PRE
II-POST-HALF
3
III-PRE
III-POST-HALF
4
IV-PRE
IV-POST-HALF
5
IV-PRE
IV-POST-ONE
6
V-PRE
V-POST-HALF
7
V-PRE
V-POST-ONE
8
VI-PRE
VI-POST-HALF
9
VI-PRE
VI-POST-ONE ...
Embodiment approach 3
[0091] Kit: a kit for detecting Hemoglobin subunit beta protein or its degradation products in body fluids or tissues such as serum or cerebrospinal fluid, which contains antibodies against Hemoglobin subunit beta protein or its degradation products.
[0092] The steps of using the above kit to detect the Hemoglobin subunit beta protein or its degradation product in the patient's serum: collect the patient's serum according to the method of embodiment 1, react with the antibody of the Hemoglobin subunit beta protein or its degradation product, and effectively detect the Hemoglobin subunit beta protein or its degradation product of the present invention The expression levels of biomarkers can effectively detect whether the subject has epilepsy, assist in the classification and diagnosis of drug-refractory epilepsy, and determine whether the drug can be reduced or discontinued after surgery for drug-resistant epilepsy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com