High potent herbicide R-napropamide and preparation method thereof
A technology of napropamide and herbicide, which is applied in the field of high-efficiency herbicide R-napropamide and its preparation, which can solve the problems of reporting the high-efficiency herbicide of napropamide, and achieve the effect of shortening the enantiomeric time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
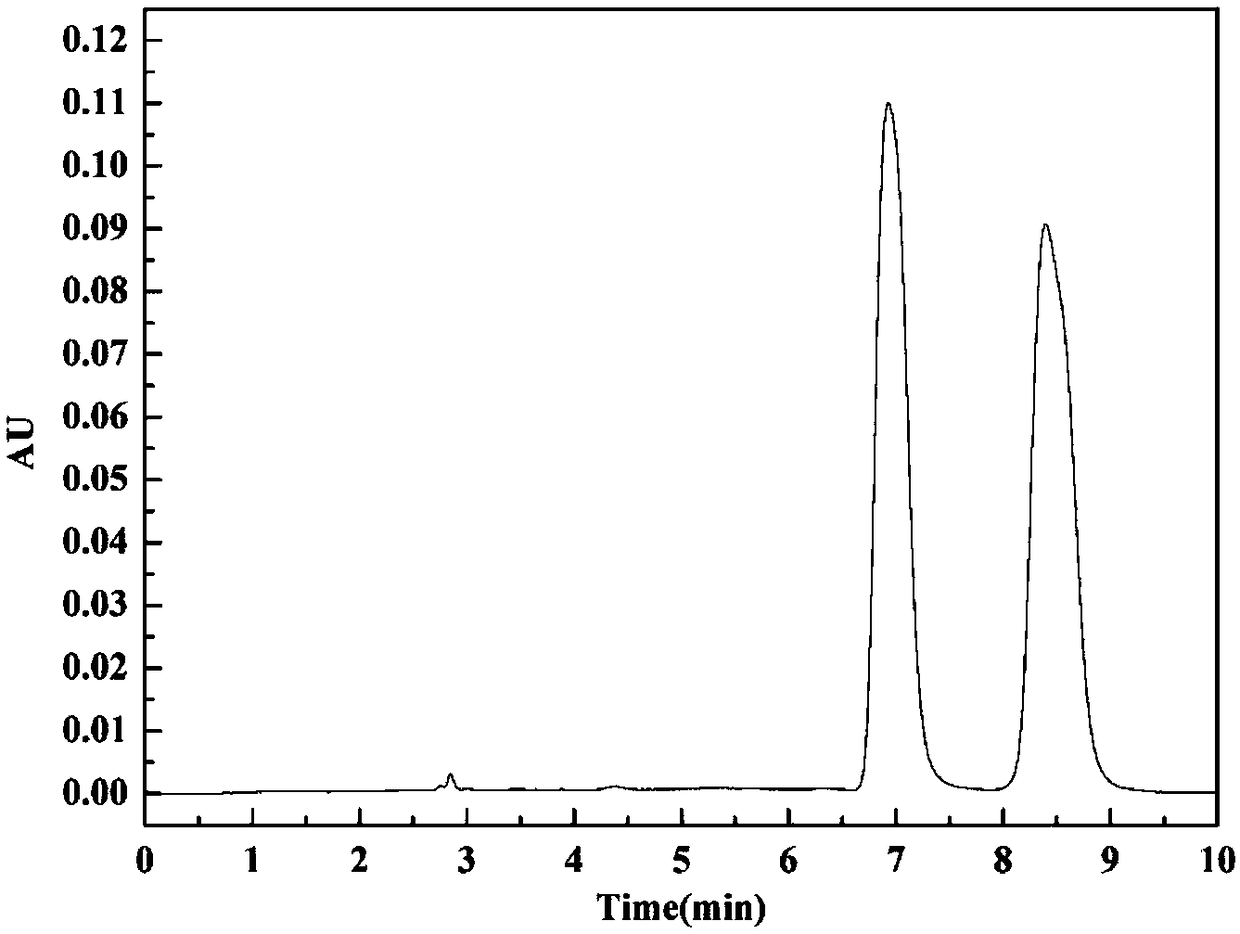
[0038] Dissolve racemic Rac-Napropamide in ethanol to prepare 1000mg L -1 stock solution, and then carry out chiral resolution to it by HPLC high-performance liquid chromatography, chromatographic column model Chiralcel OJ-H (10mm × 250mm), mobile phase n-hexane / IPA=85 / 15, flow rate 5ml min -1 , temperature 40°C. Prepare the resolution chromatogram as figure 2 As shown, there are two chromatographic peaks in the figure, indicating that two types of enantiomers pk1 and pk2 were separated.
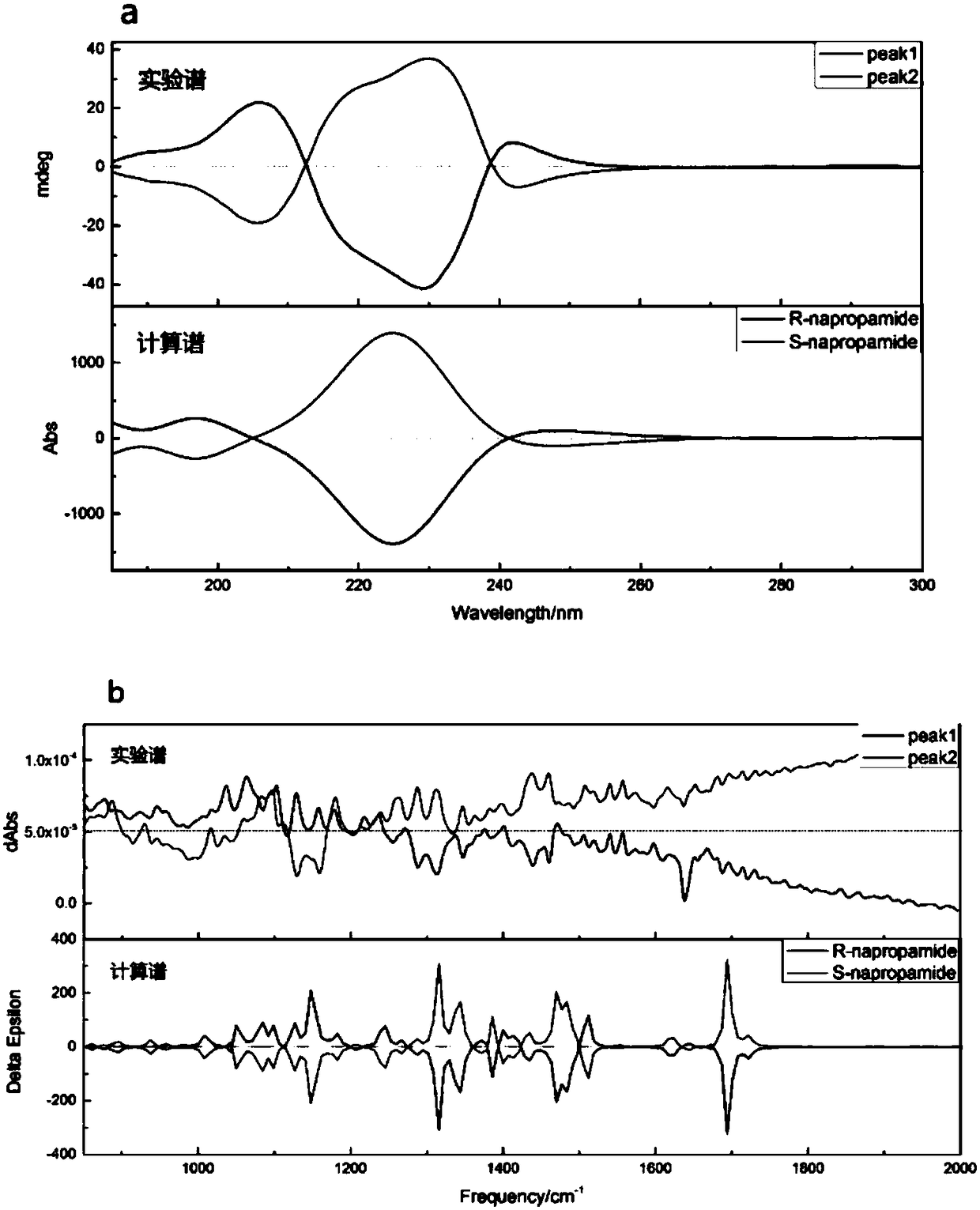
[0039] The prepared pk1 and pk2 were subjected to electronic circular dichroism (ECD) and vibrational circular dichroism (VCD) tests to obtain spectral characteristic maps of the two. Obtain the structure of R-napropamide from The PubChem Project, use computeVOA to perform conformation search, use guassian 09 to optimize the geometry of the obtained dominant conformation, calculate the circular dichroism spectrum of these dominant conformations, and then divide the spectra of each conform...
Embodiment 2
[0041] Using the R-napropamide obtained in Example 1, the target barnyardgrass was selected for herbicidal activity experiments. The experimental results showed that the R-herbicidal activity was higher than that of the S- and Rac-configurations. (table 3)
[0042] Table 3 Effect of napropamide on barnyardgrass root indicators
[0043]
[0044]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com