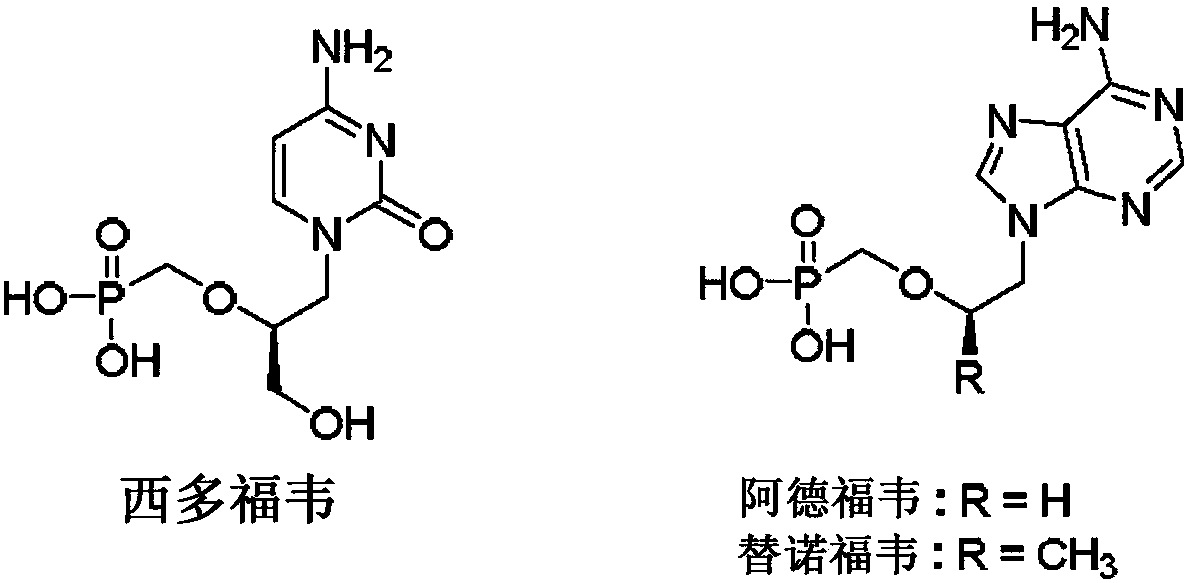
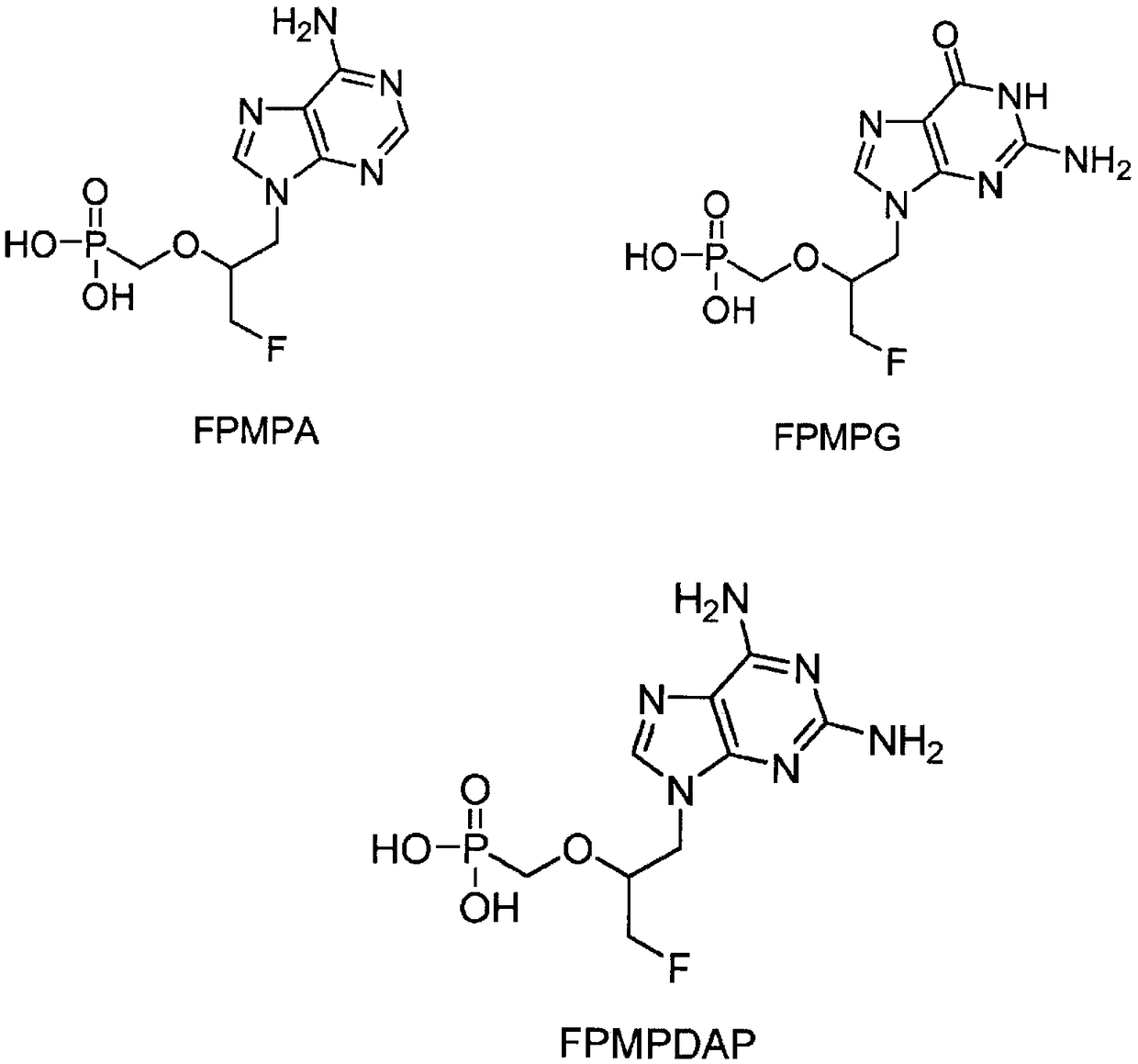
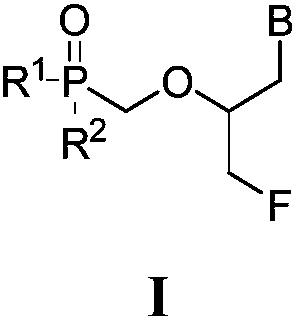
Prodrugs of fluorinated acyclic nucleoside phosphonates
A cycloalkyl compound technology, applied in the field of new aminophosphonate prodrugs, can solve the problems of impenetrability and hindering antiviral activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0159] Example 1: (S)-1-{3-fluoro-2-[(diethylphosphoryl)methoxy]propyl}-N 3 Synthesis of -(Benzyloxymethyl)thymine (6a)
[0160]
[0161] A solution of DIAD (0.32 mL, 1.64 mmol) in anhydrous THF (1 mL) was added dropwise to compound 4a at room temperature 1 (200mg, 0.82mmol), compound 5 (240mg, 0.98mmol) and Ph 3 P (430mg, 1.64mmol) in a mixture of anhydrous THF (5mL). The reaction mixture was stirred for 12 hours, then it was concentrated under reduced pressure. The crude residue was purified by silica gel column chromatography (gradient DCM / MeOH, 80:1, v / v; DCM / MeOH, 50:1, v / v) to give 6a (310 mg, 80%) as a colorless oil . 1 H NMR (300MHz, CDCl 3 ): δ7.39-7.25(m,5H,ArH),7.14-7.12(m,1H,H-6),5.50(s,2H,OCH 2 N),4.73-4.33(m,4H,CH 2 -Ar,H-3'),4.17-3.92(m,7H,H-1',H-2',2x CH 2 CH 3 ),3.84-3.66(m,2H,PCH 2 ), 1.93 (d, J=1.2Hz, 3H, CH 3 -5),1.34-1.28(m,6H,2xCH 2 CH 3 ); 13 C NMR (75MHz, CDCl 3 ): δ163.8(C-4), 151.8(C-2), 140.5(C-6), 138.1(Ar-C), 128.3(Ar-C), 127.7(Ar...
Embodiment 2
[0162] Example 2: Synthesis of (R)-1-{3-fluoro-2-[(diethylphosphoryl)methoxy]propyl}-N3-(benzyloxymethyl)thymine (6b)
[0163]
[0164] According to the procedure used for the preparation of 6a, from compound 4b 1 (300mg, 1.23mmol), Compound 5 (360mg, 1.47mmol), Ph 3 A solution of P (640 mg, 2.46 mmol) and DIAD (0.48 mL, 2.46 mmol) in anhydrous THF (8 mL) initially gave compound 6b (460 mg, 70%) as a colorless oil. The crude residue was purified by silica gel column chromatography (gradient DCM / MeOH, 80:1, v / v; DCM / MeOH, 50:1, v / v). 1 H NMR (300MHz, CDCl 3 ): δ7.39-7.25(m,5H,ArH),7.13(t,J=1.3Hz,1H,H-6),5.50(s,2H,OCH 2 N),4.73-4.33(m,4H,CH 2 -Ar,H-3'),4.17-3.91(m,7H,H-1',H-2',2x CH 2 CH 3 ),3.84-3.66(m,2H,PCH 2 ), 1.93 (d, J=1.1Hz, 3H, CH 3 -5),1.34-1.28(m,6H,2xCH 2 CH 3 ); 13 C NMR (75MHz, CDCl 3 ): δ163.8(C-4), 151.8(C-2), 140.5(C-6), 138.0(Ar-C), 128.3(Ar-C), 127.7(Ar-C), 109.7(C- 5), 82.3(d, 1 J C,F =173.5Hz, C-3'), 78.6(dd, 2 J C,F =18.5Hz, 3 J C,P =9...
Embodiment 3
[0165] Example 3: (S)-1-{3-fluoro-2-[(diethylphosphoryl)methoxy]propyl}thymine (7a)
[0166]
[0167] A suspension of compound 6a (240 mg, 0.51 mmol) and Pd / C on charcoal (10% w / w, 120 mg) in EtOH (10 mL) was purged with nitrogen and then hydrogen, then allowed to stir under an atmosphere of hydrogen. After 24 hours, the mixture was filtered through a pad of celite, and the filtrate was evaporated under reduced pressure to give the crude product. Then, the residue was redissolved in MeOH (10 mL) and Et 3 N (1 mL), and the solution was stirred at room temperature for 3 hours. The reaction mixture was concentrated under reduced pressure and the resulting crude residue was purified by silica gel column chromatography (gradient DCM / MeOH, 30:1, v / v; DCM / MeOH, 20:1, v / v) to give a colorless oil Form 7a (160 mg, 90%). 1 H NMR (300MHz, CDCl 3 ): δ9.63(s,1H,NHCO),7.16(t,J=1.3Hz,1H,H-6),4.72-4.33(m,2H,H-3'),4.17-3.90(m,7H ,H-1',H-2',2x CH 2 CH 3 ),3.83-3.63(m,2H,PCH 2 ), 1.89...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com