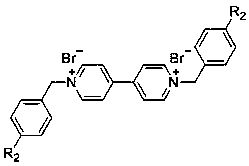
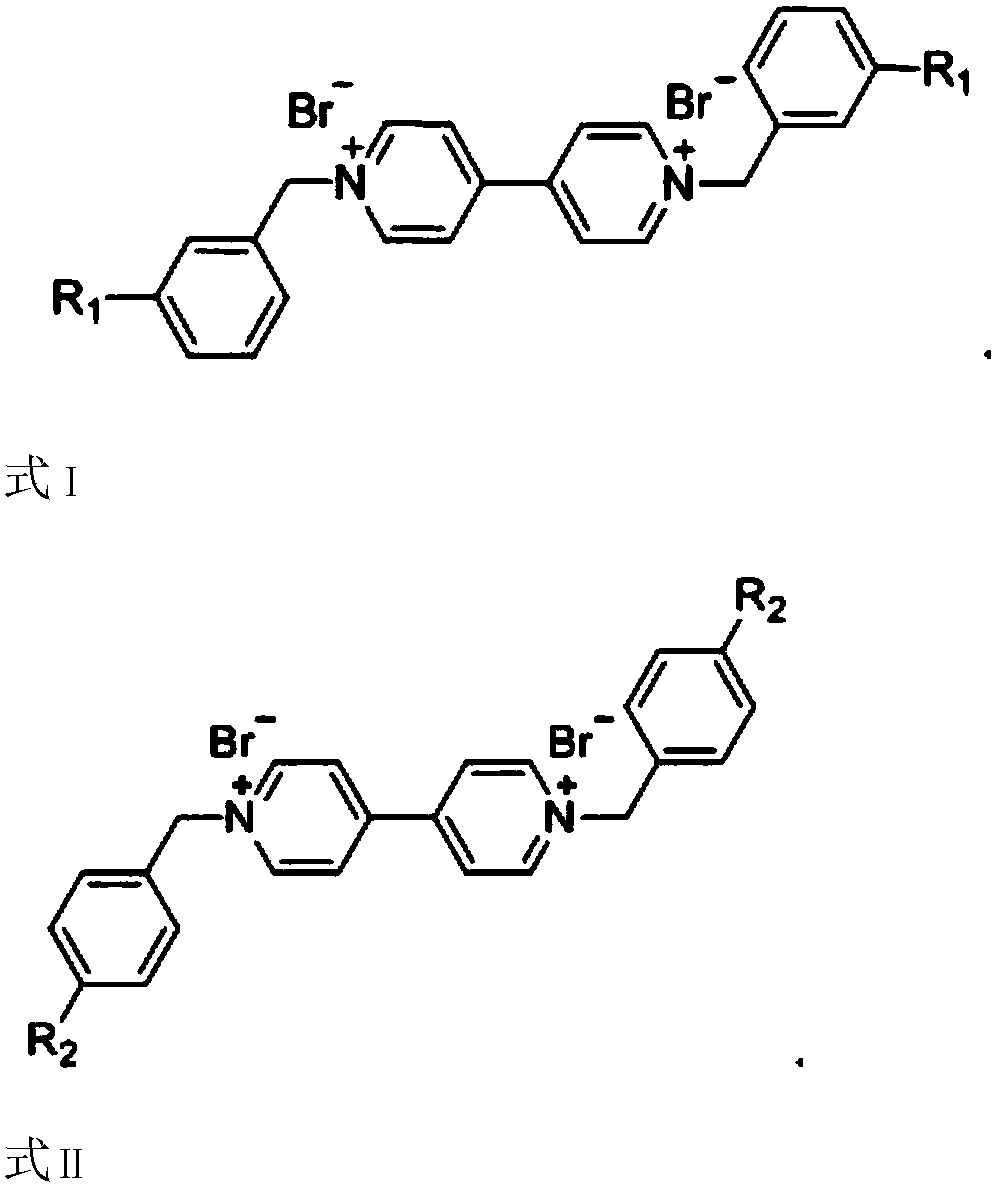
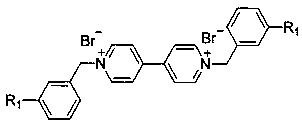Novel bifunctional viologen ionic compound and preparation method thereof
An ionic compound and bifunctional technology, which is applied in the field of new bifunctional viologen ionic compounds and preparation, can solve the problems of difficult purification of products, harsh synthesis conditions, low yields, etc., and achieves easy operation, simple process and universal applicability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019]
[0020] Preparation of viologen ion compounds c1 and c2 of formula 3 dicyano functionalization
[0021] Dicyano functionalized viologen ion compound c1: D[3-CNBzBpy]Br 2 and c2: D[4-CNBzBpy]Br 2 preparation method and characterization.
[0022] First, weigh 4,4'-bipyridine (10mmol, 1.56g) and 3-cyanobenzyl bromide (20mmol, 3.92g) in a beaker, add 30mL solvent acetonitrile, and stir to completely dissolve the raw materials. Then, the above transparent solution was transferred to a 50 mL polytetrafluoroethylene-lined stainless steel reaction kettle, placed in an oven (80-120°C), and subjected to quaternization reaction for 12-24 hours under solvothermal conditions. After the reaction, the resulting solid product was simply filtered, washed and dried to obtain a yellow solid, namely dicyano functionalized viologen ionic compound c1: D[3-CNBzBpy]Br 2 , yield 85%, purity 98%. Replace 3-cyanobenzyl bromide with 4-cyanobenzyl bromide, and use the same reaction to prepa...
Embodiment 2
[0027] Dibromophenyl functionalized viologen ion compound c3: D[3-BrBzBpy]Br 2 and c4: D[4-BrBzBpy]Br 2 preparation method and characterization.
[0028] First, weigh 4,4'-bipyridine (10mmol, 1.56g) and 3-bromobenzyl bromide (20mmol, 5.00g) in a beaker, add 30mL solvent acetonitrile, and stir to completely dissolve the raw materials. Then, the above transparent solution was transferred to a 50 mL polytetrafluoroethylene-lined stainless steel reaction kettle, placed in an oven (80-120° C.), and subjected to quaternization reaction for 12-24 hours under solvothermal conditions. After the reaction, the resulting solid product was simply filtered, washed and dried to obtain a yellow solid, i.e. the dibromophenyl functionalized viologen ion compound c3: D[3-CNBzBpy]Br 2 , yield 86%, purity 98%. Replace 3-bromobenzyl bromide with 4-bromobenzyl bromide, and use the same reaction to prepare viologen ion monomer c4: D[4-BrBzBpy]Br 2 (light yellow solid, yield 90%, purity 98%), thei...
Embodiment 3-6
[0039] Adopt the similar method of embodiment 1 and example 2, embodiment 3 uses 3-nitrobromobenzyl (3-NO 2 BzBr) and 4-nitrobenzyl bromide (4-NO 2 BzBr) was used as raw material to react with 4,4'-bipyridine to prepare two dinitrobenzene functionalized viologen ion compounds c5:D[3-NO 2 BzBpy]Br 2 and c6:D[4-NO 2 BzBpy]Br 2 , the reaction formula is shown in formula 5.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com