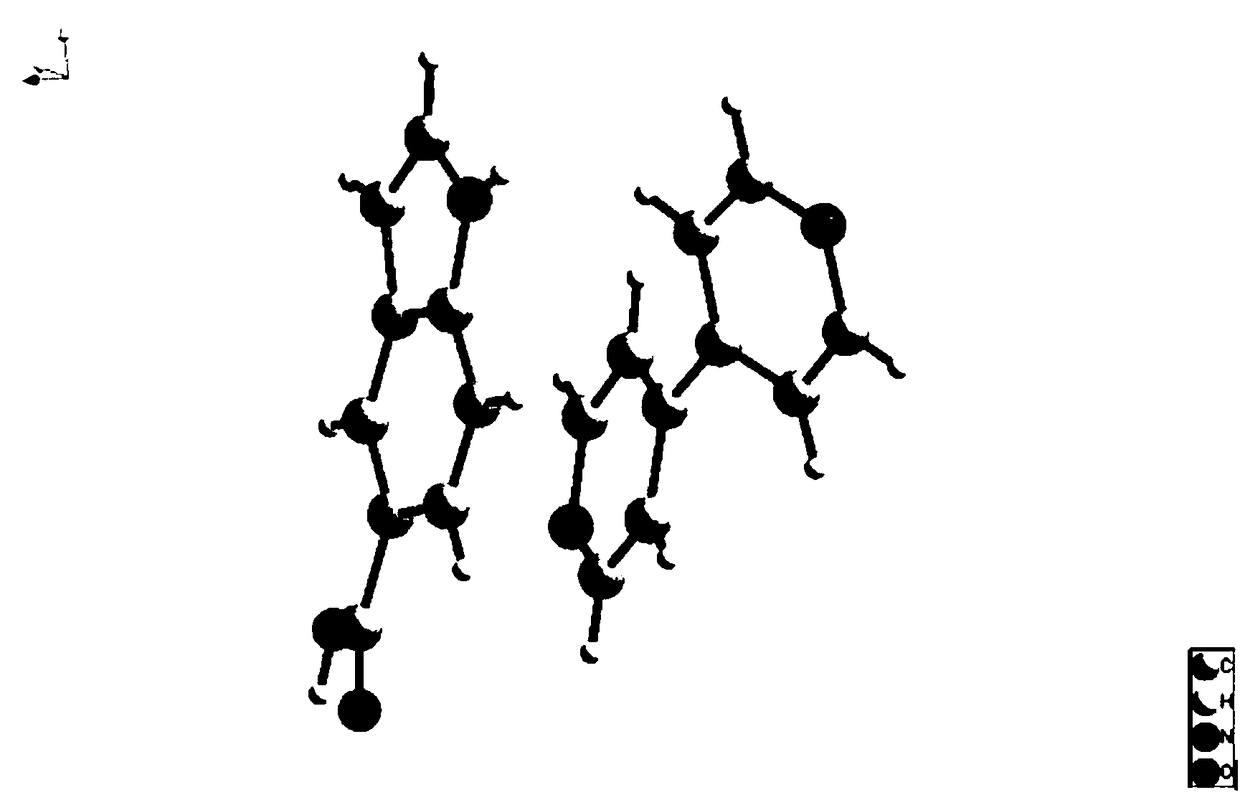
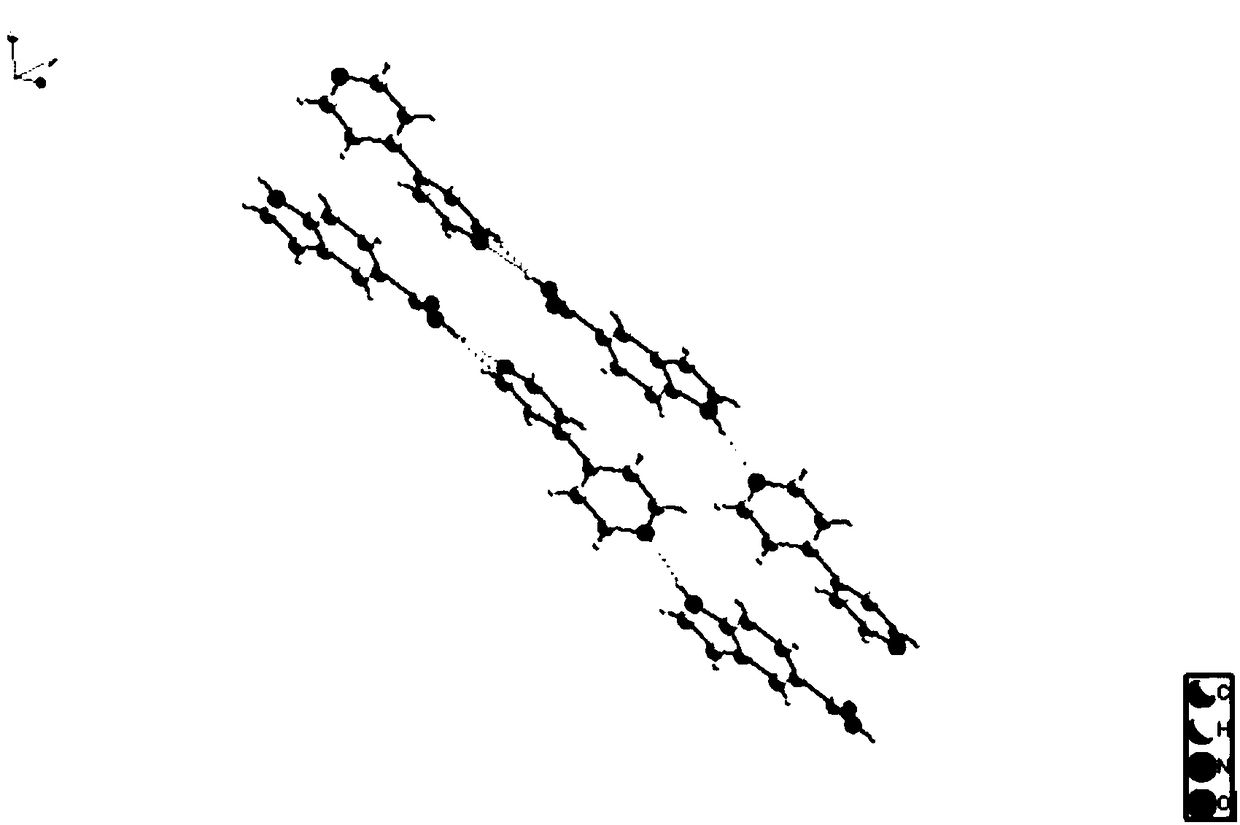
Indole-5-carboxylic acid organic pharmaceutical cocrystal and preparation method thereof
A technology of organic drugs and carboxylic acids, which is applied in the field of indole-5-carboxylic acid organic drug co-crystal and its preparation, can solve the problems of less research, achieve good application prospects, improve drug efficacy and bioavailability, physical and chemical The effect of property improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method of indole-5-carboxylic acid organic drug co-crystal:
[0033] Add 0.1mmol of indole-5-carboxylic acid and 0.1mmol of 4,4'-bipyridyl into a mixed solvent consisting of 3ml of water and 3ml of ethanol and place in a eutectic container, stir at room temperature for 2h; after the stirring stops, place the eutectic container in Place it in an oven at 80°C for 10 hours and take it out. After cooling down to room temperature, rod-shaped and block-shaped light yellow crystals are precipitated. The molar yield is 80%, and the obtained crystal particles are moderate in volume and in the form of small particles.
Embodiment 2
[0035] A preparation method of indole-5-carboxylic acid organic drug co-crystal:
[0036] Add 0.1mmol of indole-5-carboxylic acid and 0.1mmol of 4,4'-bipyridine into a mixed solvent consisting of 3ml of water and 3ml of methanol and place in a eutectic container, stir at room temperature for 2h; after the stirring stops, place the eutectic container in Place it in an oven at 80°C for 10 hours and take it out. After cooling down to room temperature, rod-shaped and block-shaped light yellow crystals are precipitated. The molar yield is 85%, and the obtained crystal particles are moderate in volume and in the form of small particles.
Embodiment 3
[0038] A preparation method of indole-5-carboxylic acid organic drug co-crystal:
[0039] Add 0.1mmol of indole-5-carboxylic acid and 0.1mmol of 4,4'-bipyridine into a mixed solvent consisting of 3ml of water and 3ml of acetone and place in a eutectic container, stir at room temperature for 2h; after the stirring stops, place the eutectic container in Place it in an oven at 80°C for 20 hours and then take it out. After cooling down to room temperature, rod-shaped and block-shaped light yellow crystals are precipitated. The molar yield is 78%, and the obtained crystal particles are moderate in volume and in the form of small particles.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com