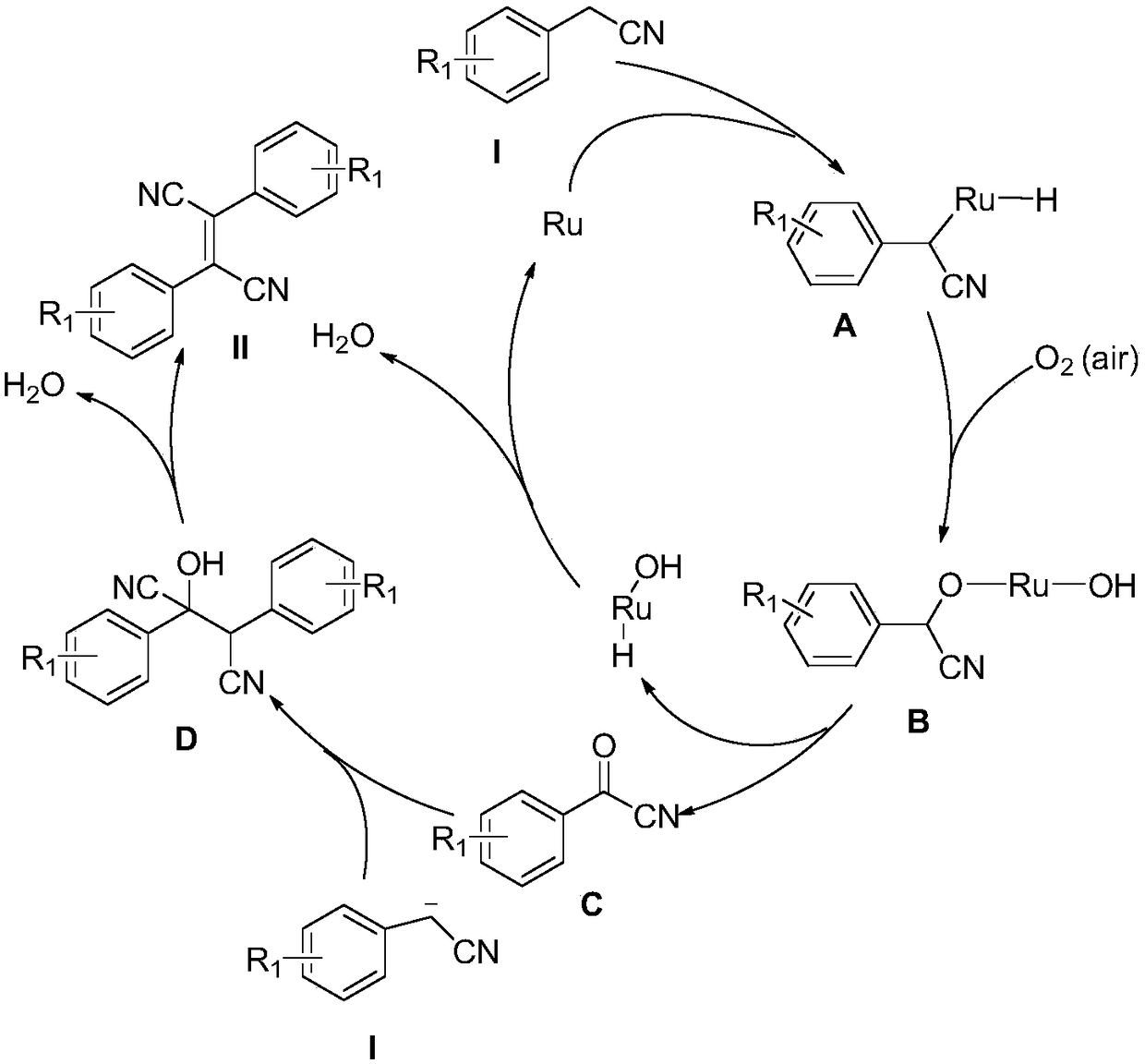
Method for synthesizing dicyano diarylethene compound
A technology of dicyanodiarylethene and synthesis method, which is applied in the preparation of organic compounds, chemical instruments and methods, organic chemistry and other directions, can solve problems such as restricting industrialization, and achieves reduction of reaction cost, pollution, and reaction bottom. Broad spectrum effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
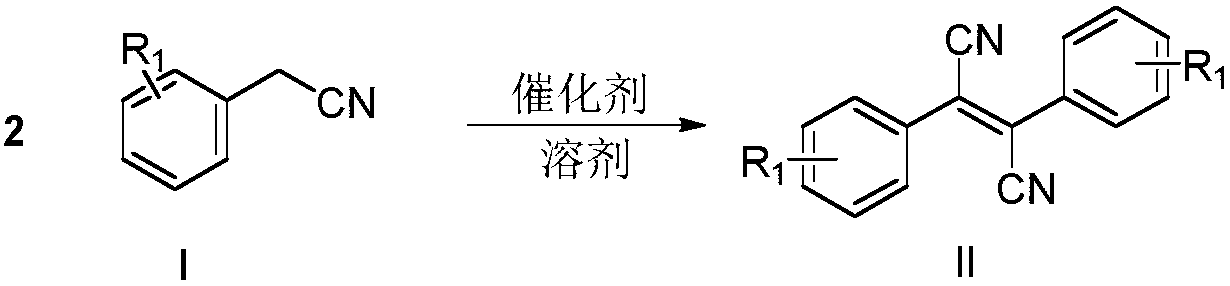
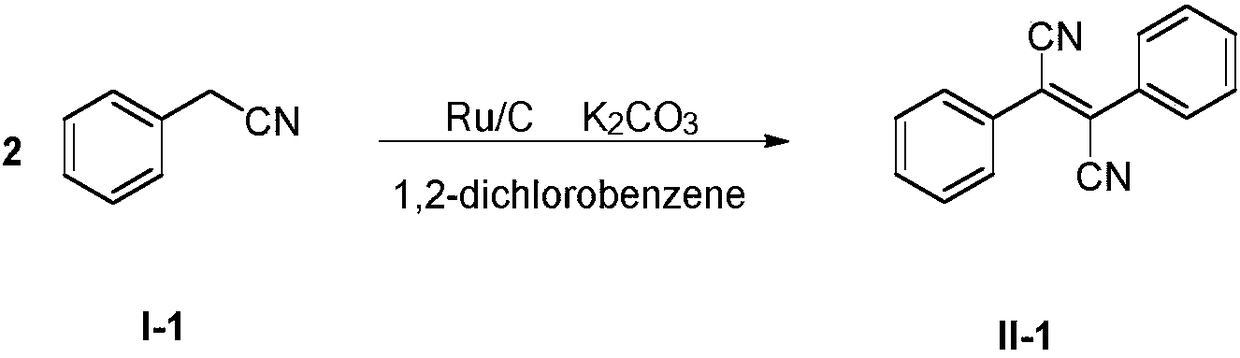
[0024] Embodiment 1: the preparation of dicyano-stilbene (II-1)
[0025] The reaction formula is as follows:
[0026]
[0027] Under air atmosphere, add 0.1g (Ru molar mass is 5‰ of (I-1)) Ru / C catalyst, 15mmol K 2 CO 3 Into the reaction flask, add 50mL o-dichlorobenzene, then add 1.172g (10mmol) phenylacetonitrile (I-1) into the reaction flask under stirring, keep the temperature at about 160°C, stir for 30 hours, TLC Monitor until the basic reaction of raw material (I-1) is complete. After the reaction, add 20mL water to the reaction solution, stir and filter once to obtain a filter cake and a filtrate, wash the filter cake once with 5mL of o-dichlorobenzene and then filter it twice, and recycle the filter cake twice to obtain Ru / C catalyst; combine the primary filtrate and the secondary filtrate, collect the organic phase after standing for stratification, and reclaim o-dichlorobenzene through distillation under reduced pressure under 10mmHg (collecting the fraction ...
Embodiment 2
[0029] Embodiment 2: the preparation of dicyano two p-chlorostyrene (II-2)
[0030] The reaction formula is as follows:
[0031]
[0032] Under air atmosphere, add 0.1g (Ru molar mass is 5‰ of (I-1)) Ru / C catalyst, 15mmol K 2 CO 3 Add 50mL o-dichlorobenzene to the reaction flask, then add 1.516g (10mmol) p-chlorophenylacetonitrile (I-2) into the reaction flask under stirring, keep the temperature at about 160°C, and stir for 30 hours , the following operations were the same as in Example 1, and finally 1.406 g of the dicyanodi-p-chlorostyrene compound shown in formula (II-2) was obtained, with a yield of 94% and a GC-MS purity of 99.0%. The structural representation of compound formula (II-2) is as follows:
[0033] 1 H-NMR (CDCl 3 ): δ7.73(d, 2H, J=6Hz), 7.51(d, 2H, J=12Hz), 7.35-7.32(m, 4H); IRν max (cm -1):3092,2224,1592,1492,1404,1257,1093,1012,826,824; GC-MS(EI):m / z 298[M + ]
Embodiment 3
[0034] Embodiment 3: the preparation of dicyano two-trifluoromethylstyrene (II-3)
[0035] The reaction formula is as follows:
[0036]
[0037] Under air atmosphere, add 0.1g (Ru molar mass is 5‰ of (I-1)) Ru / C catalyst, 15mmol K 2 CO 3 In the reaction flask, add 50mL o-dichlorobenzene, then add 1.852g (10mmol) m-trifluoromethylphenylacetonitrile (I-3) into the reaction flask under stirring, keep the temperature at about 160°C, stir After reacting for 35 hours, the following operations were the same as those in Example 1. Finally, 1.593 g of the dicyanodi-trifluoromethylstyrene compound represented by formula (II-3) was obtained, with a yield of 87% and a GC-MS purity of 99.0%. The structural representation of compound formula (II-3) is as follows:
[0038] 1 H-NMR (CDCl 3 ):δ8.12(s,2H),7.92(m,2H),7.69(m,2H),7.54(m,2H); 13 C-NMR (CDCl 3 ): δ141.58, 133.41, 132.03, 130.23, 129.21, 125.54, 124.12, 122.15, 115.68; IRν max (cm -1 ):2226,1333,1171,1132,803,697; GC-MS(E...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com