A kind of preparation method of ketoxime
A technology of ketoxime and ionic liquid, applied in the field of ketoxime, can solve the problems of large alkali consumption, equipment and environmental impact, large consumption, etc., achieve high product yield and purity, mild reaction conditions, and increase nucleophilic ability Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In the three flasks, diphenyl ketone and hydroxylamine hydroxylamine were mixed by 1: 1.1-1: 1.3, and the appropriate amount of ethanol was added to completely dissolve, then add a quantity [p 4444 ] [4-ch 3 IM] (the amount of substance is 10% -50% of hydroxylamine hydrochloride), heated reflux, using thin layer chromatography (the opening agent as petroleum ether: ethyl acetate = 4: 1) Monitor the reaction process. After the reaction, the solvent ethanol is rotated, and the appropriate amount of deionized water is added, stirring, precipitating the solid, filtered and washed with a small amount of deionized water, to give a white solid, 60 ° C drying 24 h, 20%; The chromatography (mobile phase acetonitrile: water = 7: 3, UV detection wavelength is 254 nm) to determine the purity of 98% (area belonging). It is characterized by IR, NMR and MS, which is de-amphenylphenyl oxime, and melting melting point MP: 143.1-143.8 ° C.
Embodiment 2- Embodiment 23
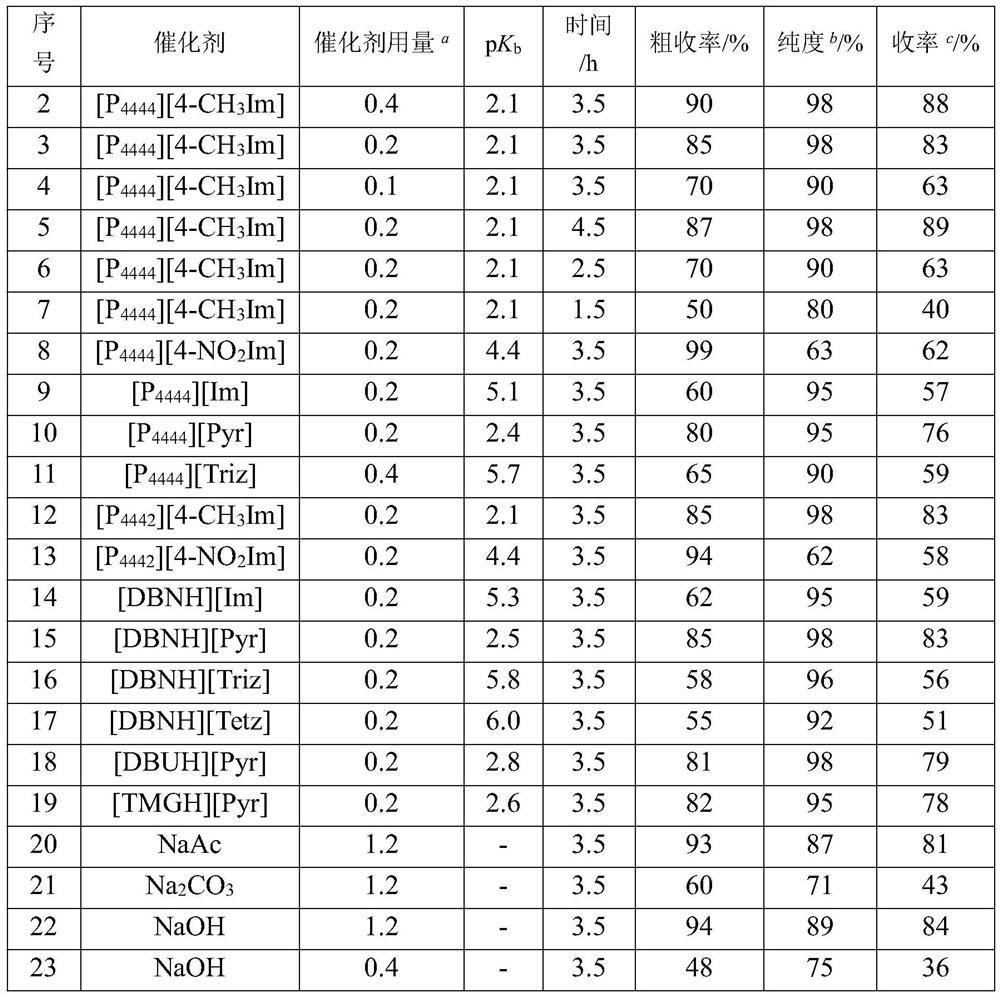
[0030]The same embodiments are used in Example 1, which distinguishes that the catalyst type and amount, azole anionic ion-functional ionic liquid alkali is as shown in the following table:
[0031]
[0032] a Compared to the amount of hydroxylamine substance of hydrochloride; b HPLC detection, mobile phase acetonitrile: water = 7: 3, area belonging; c It is obtained based on purity and crude yield.
[0033] As indicated, the azole anion-functionalized ionic liquid has a strong alkaline, efficiently catalyzed diphenyl ketone and hydrochloride reaction to prepare diphenylphenyl oxime, combined with the acid and dehydrated agent. The stronger the alkaline is, and the hydroxylamine reaction of hydroxylamine is better than the hydrochloride reaction. At the same time, the alkalineness of the azol-based anion functionalized ionic liquid is found is mainly related to the type and push of the anion, and the electron absorption is less affected by the cation. NAAc, NA with traditional h...
Embodiment 24
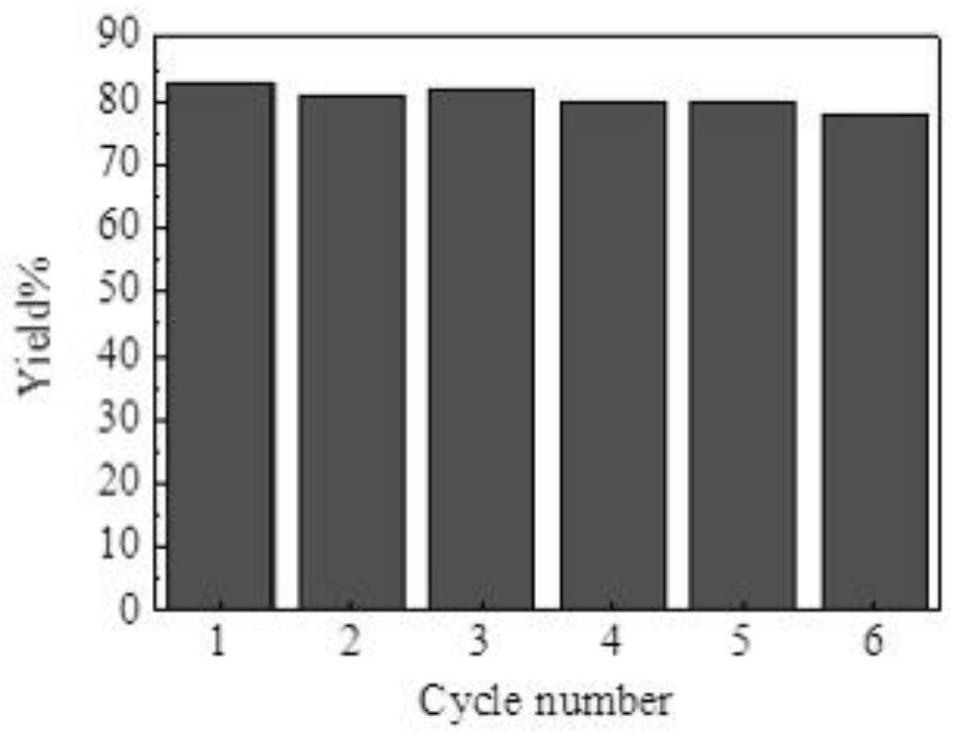
[0034] Example 24 Cycling Performance
[0035] [P 4444 ] [4-ch 3 IM] is a catalyst, 20% (mole percent) of hydroxylamine hydrochloride, hydroxylamine hydrochloride: a molar ratio of 1.2: 1, refluxed with ethanol for a solvent 3.5H, and diphenyl ketoxime yield optimal Can reach 83%.
[0036] The filtrate obtained by separating the solid product after the reaction is separated, rotationally evaporated to remove water, resulting in [P 4444 ] [4-ch 3 Im] Ion liquid, then washed 3 times with ethyl acetate, dried in vacuo at 50 ° C, and used as a catalyst for dibenzophenone and hydroxylamine hydrochloride to prepare ketoxime. After the cycle is used 6 times, the catalytic effect has not shown significant decline, such as figure 1 Indicated.
[0037] When preparing ketone oxime in conventional hydroxylamine, due to NaOH and NA 2 CO 3 The substance is neutralized with hydrochloric acid, thereby losing the cycling performance. The azol-based anion-functionalized ionic liquid used in the pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com