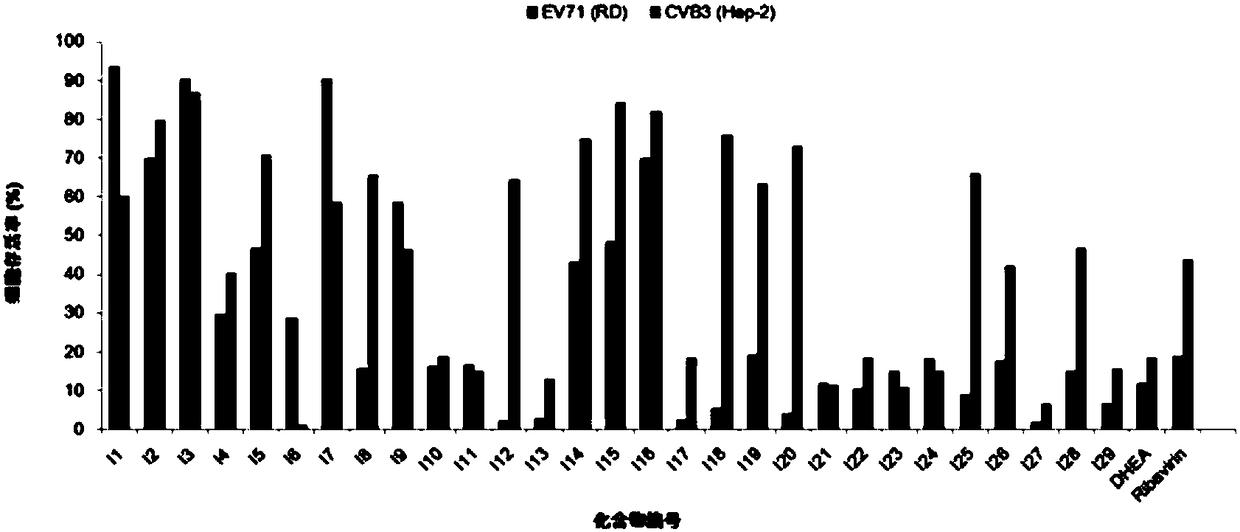
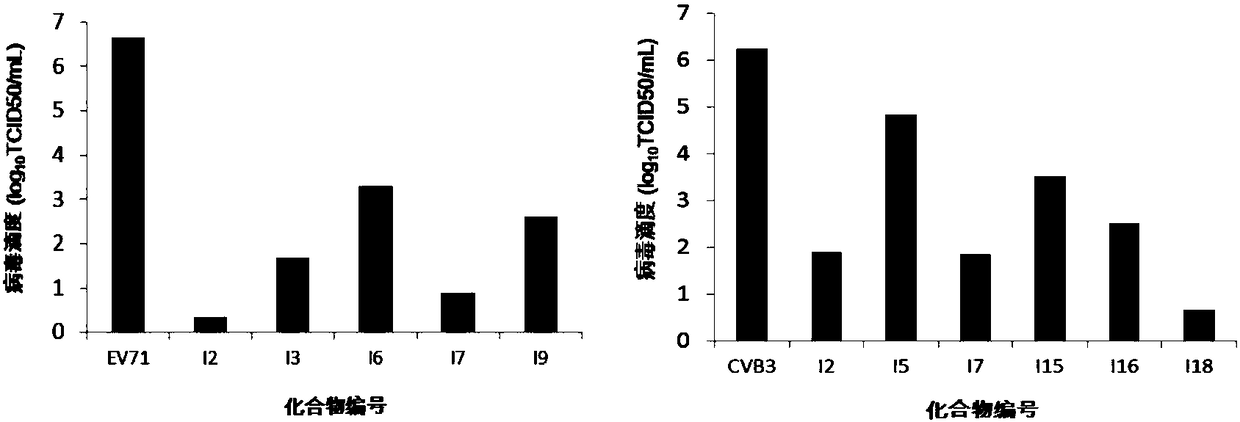
Thiazole heterocyclic ring-containing steroid derivative as well as preparation method and application thereof
A technology of derivatives and steroids, applied in the fields of organic synthesis and medicinal chemistry, can solve the problems of lack of specific and strong drugs, and achieve the effects of easy-to-obtain raw materials, good anti-viral activity, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] [Example 1] Preparation of the compound numbered as I1 in table 1
[0038] Its reaction formula is:
[0039]
[0040] Its preparation method comprises the following steps:
[0041] 1) With 5mmol of dehydroepiandrosterone (compound 1) and 5.5mmol of thiosemicarbazide (NH 2 CSNHNH 2 ) as the starting material, the intermediate 2a was prepared by condensation reaction in absolute ethanol (30mL), the reaction temperature was 70-80°C, and the reaction time was 8h;
[0042] 2) The intermediate 2a (1mmol) and α-bromoacetophenone (1mmol) were subjected to a heterocyclization reaction in absolute ethanol (15mL) to obtain the target compound I1, the reaction temperature was 60-70°C, and the reaction time was 9h. Spectral data of target compound I1: mp 242-244°C; 1 H NMR (400MHz, CDCl 3 ):δ=8.14(bs,1H),7.75(d,J=8Hz,2H),7.36(t,J=8Hz,2H),7.27(d,J=8Hz,1H),6.81(s,1H) ,5.35(d,J=4.4Hz,1H),3.53(bs,1H),2.42-2.21(m,4H),2.10-2.02(m,2H),1.95-1.75(m,3H),1.56-1.35 (m,5H),1.25-0.93(m,1...
Embodiment 2
[0043] [Example 2] Numbering in table 1 is the preparation of the compound of I12
[0044] Its reaction formula is:
[0045]
[0046] Its preparation method comprises the following steps:
[0047] 1) With 3mmol of dehydroepiandrosterone (compound 1) and 3.3mmol of N-methylthiosemicarbazide (CH 3 NHCSNHNH 2 ) as the starting material, the intermediate 2b was prepared by condensation reaction in anhydrous methanol (20mL), the reaction temperature was 60-70°C, and the reaction time was 7h;
[0048] 2) Heterocyclization reaction of intermediate 2b (1mmol) and α-bromoacetophenone (1mmol) in anhydrous acetonitrile (10mL) to obtain the target compound I12, the reaction temperature is 65-75°C, the reaction time for 8h. Spectral data of the target compound I12: mp 206-208°C; 1 H NMR (400MHz, CDCl 3 ):δ=12.40(bs,1H),8.14(bs,1H),7.52-7.46(m,3H),7.35(q,J=8Hz,2H),6.63(s,1H),5.35(d,J =4Hz,1H),4.01(s,3H),3.52(bs,1H),3.35-2.90(m,2H),2.35-1.65(m,10H),1.55-1.35(m,4H),1.30-0.97 (m,9H)...
Embodiment 3
[0049] [embodiment 3] the preparation of the compound numbered as I21 in table 1
[0050] Its reaction formula is:
[0051]
[0052] Its preparation method comprises the following steps:
[0053] 1) With 6mmol of dehydroepiandrosterone (compound 1) and 6.6mmol of N-phenylthiosemicarbazide (PhNHCSNHNH 2 ) as the starting material, the intermediate 2c was prepared by condensation reaction in anhydrous methanol (40mL), the reaction temperature was 65-70°C, and the reaction time was 8h;
[0054] 2) The intermediate 2c (1mmol) and α-bromoacetophenone (1mmol) were subjected to a heterocyclization reaction in absolute ethanol (10mL) to obtain the target compound I21, the reaction temperature was 75-80°C, and the reaction time was 7.5h. Spectral data of the target compound I21: mp 173-175°C; 1 H NMR (400MHz, CDCl 3 ):δ=12.12(bs,1H),7.60-7.20(m,8H),7.02(d,J=8Hz,2H),6.67(s,1H),5.32(s,1H),3.51(bs,1H ),3.20-2.60(m,2H),2.40-1.75(m,7H),1.55-1.35(m,6H),1.30-0.95(m,10H); MS(ESI)m / z 5...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com