A kind of preparation method of high-purity fluocinolone
A technology of fluocinolone and water purification, applied in the fields of steroids, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0028] In order to solve the above-mentioned technical problems, the invention provides a kind of preparation method of high-purity fluocinolone, the preparation method of described high-purity fluocinolone comprises the following steps:
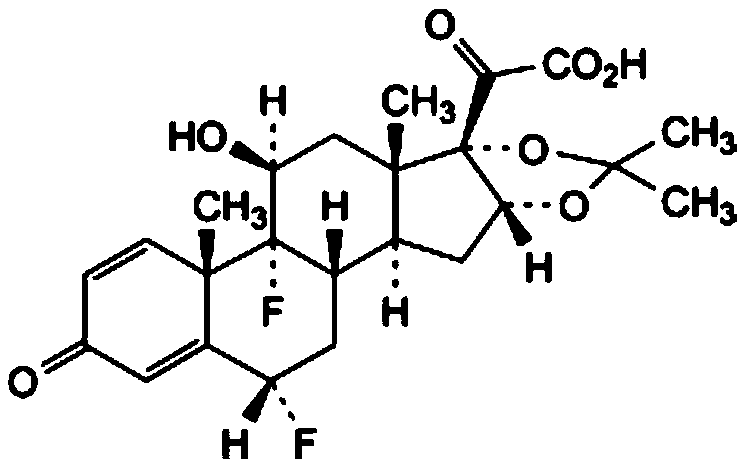
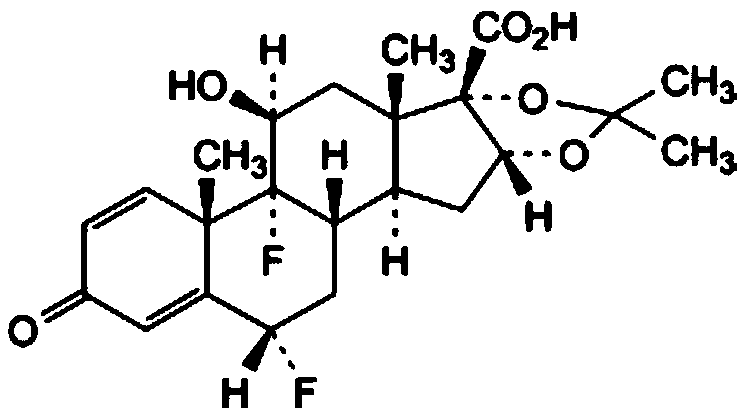
[0029] (1) Add 11β-hydroxyl-16α,17-[(1-methylethylene)-bis(oxygen)]-21-(acetyloxy)-6α,9-difluoropregna- 1,4-diene-3,20-dione, the first organic solvent, pass in an inert gas, stir; cool down to 0-5°C, add a hydrolysis reagent, keep warm for 4-10 hours, adjust the pH with acetic acid after the reaction When the value reaches 6-7, heat up to 35-45°C, concentrate under reduced pressure to 10-35% of the weight of the solution before concentration, add purified water dropwise, and keep stirring at 5-15°C for 1-3h; filter and use the mass fraction Rinse with 30-70% ethanol, and dry under reduced pressure at 60°C to obtain 11β, 21-dihydroxy-16α,17-[(1-methylethylene)-bis(oxygen)]-6α,9- Crude difluoropregna-1,4-diene-3,20-dione;
[0030](2) Add 11...
Embodiment 1
[0084] The preparation method of described high-purity fluocinolone comprises the following steps:
[0085] (1) Add 11β-hydroxyl-16α,17-[(1-methylethylene)-bis(oxygen)]-21-(acetyloxy)-6α,9-difluoropregna- 1,4-diene-3,20-dione, the first organic solvent, pass inert gas, stir; cool down to 2°C, add hydrolysis reagent, keep warm for 6 hours, after the reaction, adjust the pH value to 6- 7. Heat up to 40°C, concentrate under reduced pressure to 30% of the weight of the solution before concentration, add purified water dropwise, and keep stirring at 10°C for 2 hours; filter, rinse with ethanol with a mass fraction of 50%, and reduce Press and dry to get 11β, 21-dihydroxy-16α, 17-[(1-methylethylene)-bis(oxygen)]-6α, 9-difluoropregna-1,4-diene-3, 20-diketone crude product; the first organic solvent is absolute ethanol; the inert gas is nitrogen; the 11β-hydroxyl-16α, 17-[(1-methylethylene)-bis(oxygen)] The weight ratio of -21-(acetyloxy)-6α,9-difluoropregna-1,4-diene-3,20-dione to ...
Embodiment 2
[0089] The preparation method of described high-purity fluocinolone comprises the following steps:
[0090] (1) Add 11β-hydroxyl-16α,17-[(1-methylethylene)-bis(oxygen)]-21-(acetyloxy)-6α,9-difluoropregna- 1,4-diene-3,20-dione, the first organic solvent, pass inert gas, stir; cool down to 2°C, add hydrolysis reagent, keep warm for 6 hours, after the reaction, adjust the pH value to 6- 7. Heat up to 40°C, concentrate under reduced pressure to 30% of the weight of the solution before concentration, add purified water dropwise, and keep stirring at 10°C for 2 hours; filter, rinse with ethanol with a mass fraction of 50%, and reduce Press and dry to get 11β, 21-dihydroxy-16α, 17-[(1-methylethylene)-bis(oxygen)]-6α, 9-difluoropregna-1,4-diene-3, 20-diketone crude product; the first organic solvent is absolute ethanol; the inert gas is nitrogen; the 11β-hydroxyl-16α, 17-[(1-methylethylene)-bis(oxygen)] The weight ratio of -21-(acetyloxy)-6α,9-difluoropregna-1,4-diene-3,20-dione to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com