Pyrethroid compound containing difluorochrysanthemic acid structure
A technology of pyrethroid and perfluthrin is applied in the field of pyrethroid compounds and can solve problems such as difficulty in costing new pyrethroid compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
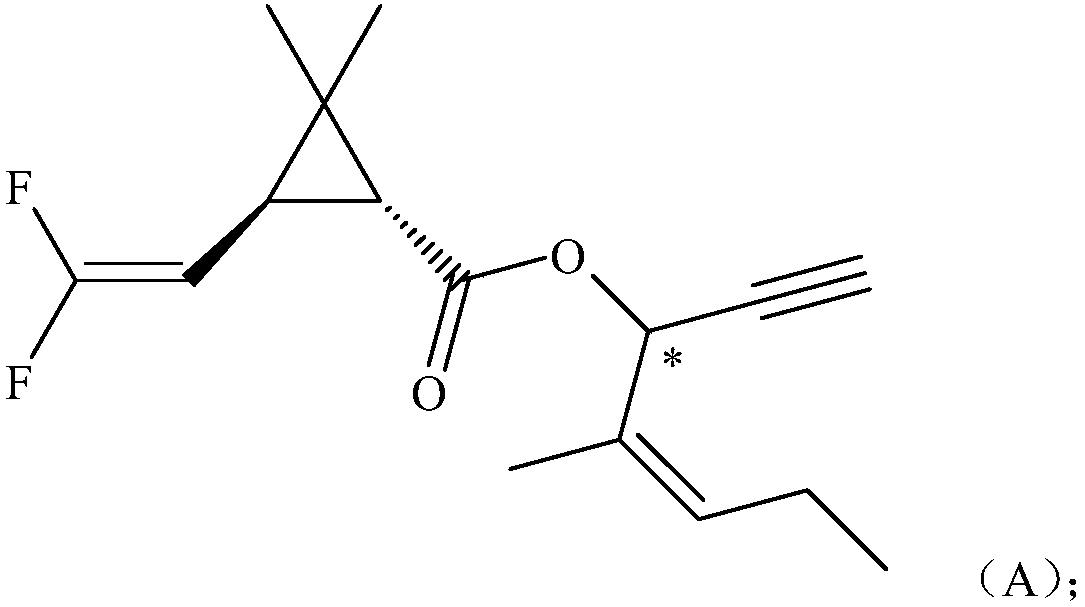
[0043] Example 1: Compound A1 of the present invention 2,2-dimethyl-1R-trans-3-(2,2-difluorovinyl)cyclopropanecarboxylic acid-1-ethynyl-2-methylpenta-2 -Synthesis of alkenyl esters
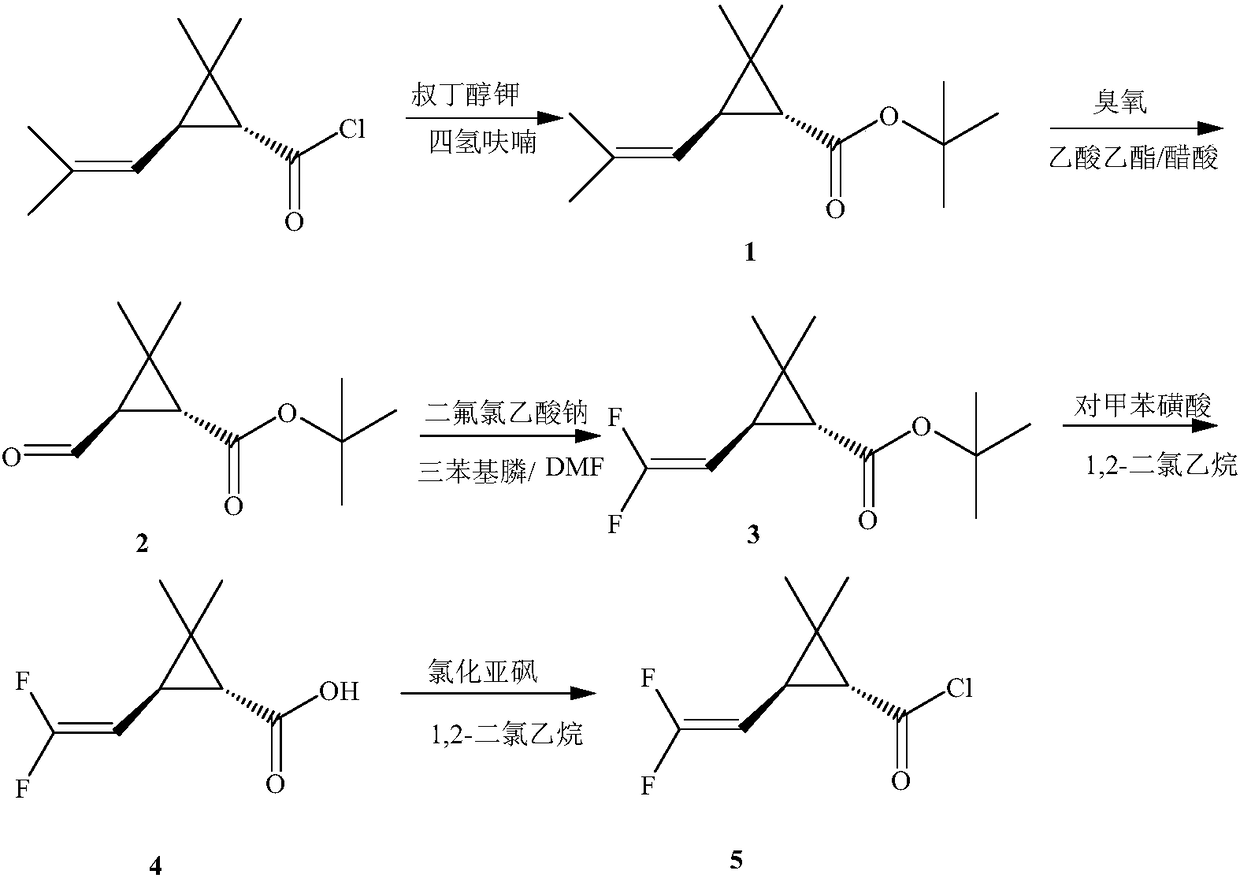
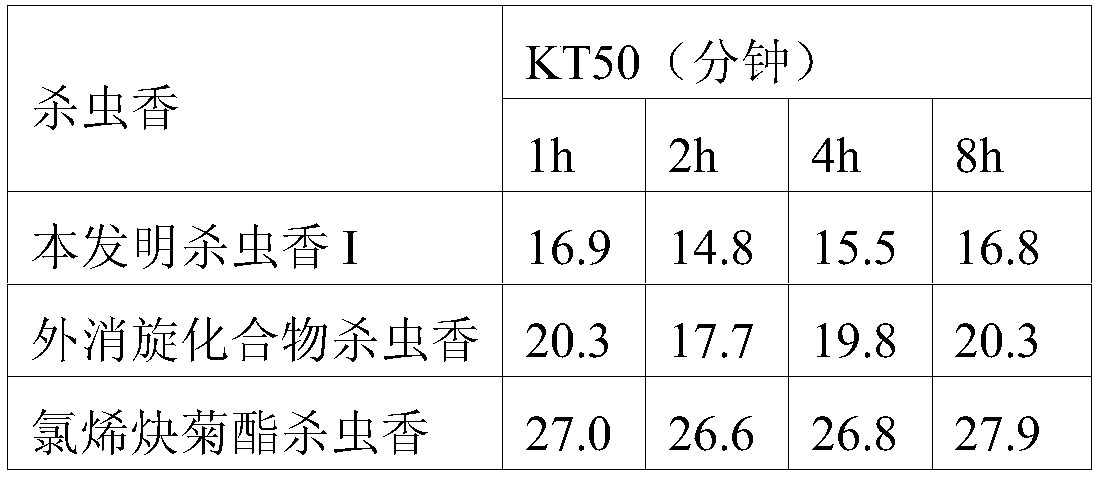
[0044]In a 200ml four-necked bottle, put 6.2g (0.05mol) of 4-methyl-1-yne-4-ene-3-heptanol, 5.0g of pyridine, dissolve in 60ml of dichloroethane, and stir , 9.73g (0.05mol) of 2,2-dimethyl-1R-trans-3-(2,2-difluorovinyl)cyclopropanecarboxylic acid chloride was added dropwise at 0~5°C, and the dropwise temperature rose to 20°C React for 4 hours. Wash with 400ml of 5% hydrochloric acid, and then with 400ml of 5% NaHCO3 Wash, separate the oil layer and heat it to 60°C under a negative pressure of 10mmHg to remove the solvent dichloroethane to obtain the compound 2,2-dimethyl-1R-trans- 3-(2,2-Difluorovinyl)cyclopropanecarboxylic acid-1-ethynyl-2-methylpent-2-enyl ester, after column chromatography treatment, the weight was 11.2g light yellow oily liquid, the content was 98.0%, yield 71.3%. The mole...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com