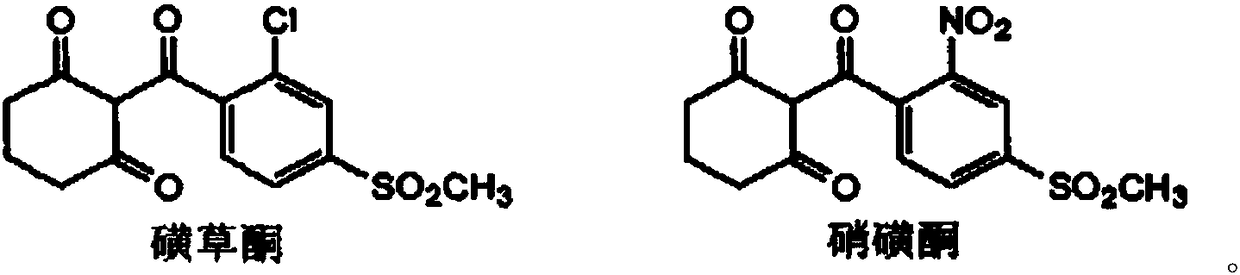
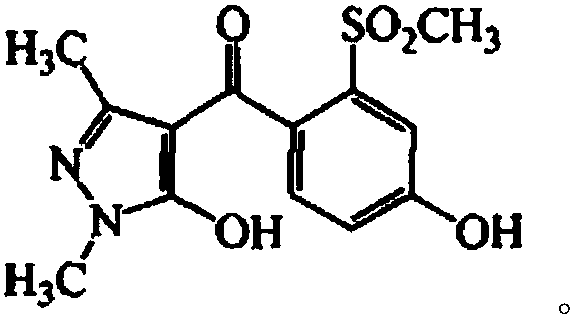
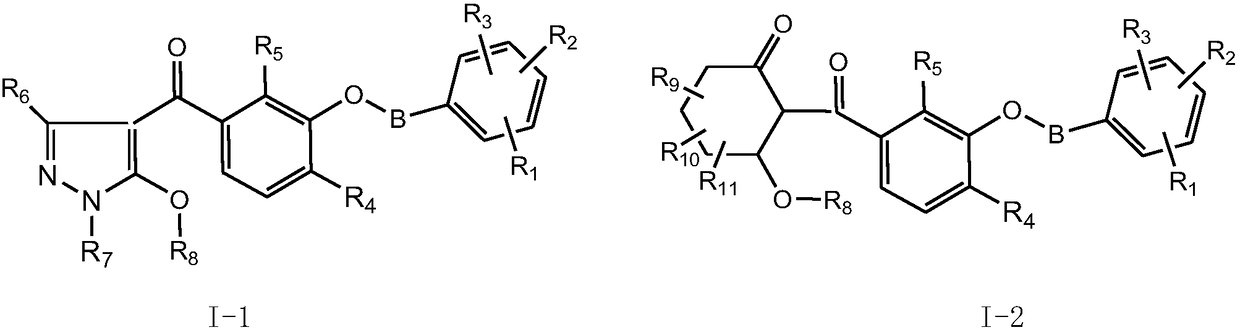
Substituted phenyl ketone compounds with ether structure, and preparation method and application thereof
A compound, phenyl ketone technology, applied in the field of phenyl ketone compounds, can solve the problems of undisclosed compounds, activity of undisclosed compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0166] Example 1, 2-methyl-4-thiamphenyl-3-(2-(p-tolyloxy)ethoxy)benzoic acid 1,3-dimethyl-1-H-pyrazole-5-ester Synthesis of (III-1)
[0167] 0.20 moles of 2-methyl-4-thiamphenyl-3-(2-(p-tolyloxy)ethoxy)benzoyl chloride (I-1) was dissolved in tetrahydrofuran, and it was added dropwise to 1, In a mixed solution of 0.20 moles of 3-dimethyl-1-H-5-hydroxypyrazole (II-1), 0.24 moles of triethylamine and tetrahydrofuran, continue to stir for 2 hours, filter, concentrate and remove the mother liquor to obtain compound 2 -Methyl-4-thiamphenyl-3-(2-(p-tolyloxy)ethoxy)benzoic acid 1,3-dimethyl-1-H-pyrazole-5-ester (III-1) .
[0168] In the same way, various intermediates (III) can be synthesized using different starting materials (I) and (II).
[0169] In the same way, various intermediates (VI) can be synthesized using different starting materials (I) and (V).
Embodiment 2
[0170] Example 2, (5-hydroxyl-1,3-dimethyl-1-H-pyrazole-4-)(2-methyl-4-thiamphenicol-3-(2-(p-tolyloxy) Synthesis of Ethoxyphenyl) Ketone (IV-1)
[0171] Dissolve 0.2 moles of compound (III-1) obtained in Example 1 in acetonitrile, add 0.28 moles of triethylamine and 0.02 moles of acetone cyanohydrin, continue stirring at room temperature for 12 hours, TLC detects that the transposition reaction is complete, concentrate and remove the solvent, add Dissolve in water, acidify to PH=2 with 1N dilute hydrochloric acid, extract with dichloromethane and dry to obtain compound (IV-1).
[0172] In the same way, various intermediates (IV) can be synthesized using different starting materials (III).
[0173] In the same way, various intermediates (VII) can be synthesized using different starting materials (VI).
Embodiment 3
[0174] Embodiment 3, the preparation of compound E-166
[0175] 0.010 moles of compound (IV-1) obtained in Example 2 and 0.012 moles of triethylamine were dissolved in tetrahydrofuran, and the tetrahydrofuran solution of 0.010 moles of sec-butyl chloroformate was added dropwise under ice-cooling, and stirring was continued at room temperature for 4 hours, and TLC detected that After the reaction was complete, it was filtered, concentrated and desolvated, and then separated by silica gel chromatography to obtain compound E-166.
[0176] In the same way, various compounds in which the substituent X is a direct bond can be synthesized using different starting materials (IV) and chloroformate.
[0177] In the same way, various compounds in which the substituent X is a direct bond can be synthesized using different starting materials (VII) and chloroformate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com