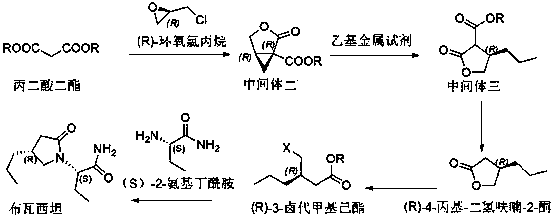
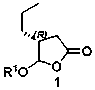
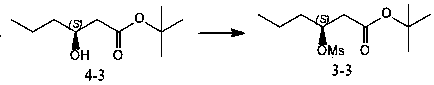Brivaracetam chiral intermediate and preparing method thereof
A technology for chiral intermediates and compounds, applied in the field of pharmaceutical synthesis, can solve the problems of cumbersome steps, high production cost, poor industrial feasibility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] In order to better understand the present invention, the following will be described in detail through specific examples. It should be noted that the following examples are not limitations of the present invention. Obviously, those of ordinary skill in the art can understand the present invention within the scope of the present invention according to the description herein. Various modifications and changes are made to the invention, and these corrections and changes are also included in the scope of the present invention.
[0037] Example 1 (preparation of formula 6 compound (S)-1-chloromethyl-1-butanol)
[0038] In the reaction flask, add 350.0g (3.78mol) of S-epichlorohydrin, 5.4g (37.8mmol) of cuprous bromide, and 3.5L of tertiary methyl ether under the protection of argon, and lower the temperature to -78~-80°C. Control the internal temperature at -78~-80°C and add 756mL (3.78mol) of 5.0 mol / L ethylmagnesium chloride tetrahydrofuran solution dropwise, and keep the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com