2,4,6-triamino-5-nitropyrimidine-1,3-dioxide and nitric acid self-assembled crystal and preparation method thereof
A technology of nitropyrimidine and dioxide is applied in the directions of nitrated acyclic/alicyclic/heterocyclic amine explosive compositions, attack equipment, explosives processing equipment, etc., and can solve the problems of no public literature report, sparse research, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
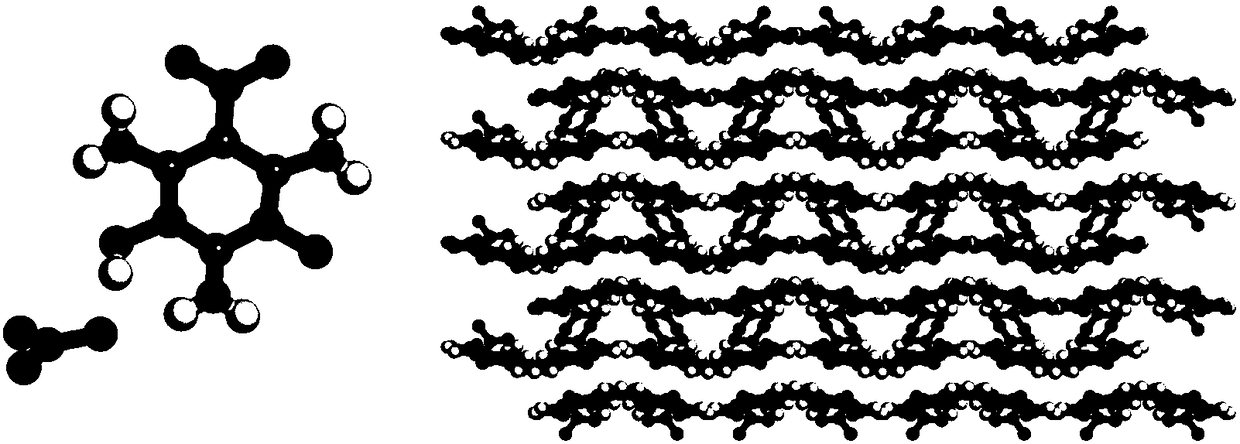
[0022] At 20°C, add 10ml of 5% nitric acid aqueous solution into a three-necked flask, then heat a sufficient amount of 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide under stirring to dissolve , and filtered to obtain a saturated aqueous solution of 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide in nitric acid. Put the filtrate in a beaker, then put it still in a thermostat at 25°C, let the solvent evaporate, precipitate crystals, and dry to obtain 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide and nitric acid Self-assembled energetic crystals, the structure of which is attached figure 1 shown.
Embodiment 2
[0024] At 20°C, add 10ml of 30% nitric acid aqueous solution into a three-necked flask, then heat a sufficient amount of 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide under stirring to dissolve , and filtered to obtain a saturated aqueous solution of 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide in nitric acid. Put the filtrate in a beaker, then put it still in a thermostat at 25°C, let the solvent evaporate, precipitate crystals, and dry to obtain 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide and nitric acid Self-assembled energetic crystals.
Embodiment 3
[0026] At 20°C, add 10ml of 60% nitric acid aqueous solution into a three-necked flask, then heat a sufficient amount of 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide under stirring to dissolve , and filtered to obtain a saturated aqueous solution of 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide in nitric acid. Put the filtrate in a beaker, then put it still in a thermostat at 35°C, let the solvent evaporate, precipitate crystals, and dry to obtain 2,4,6-triamino-5-nitropyrimidine-1,3-dioxide and nitric acid Self-assembled energetic crystals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com