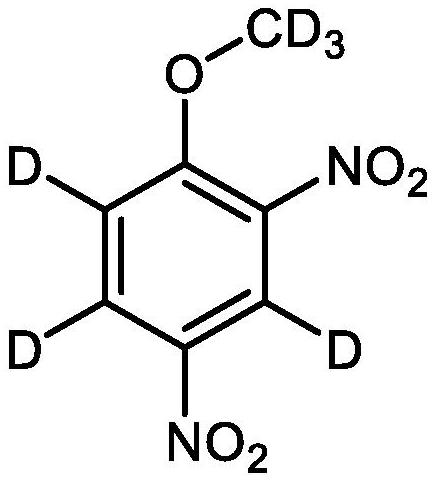
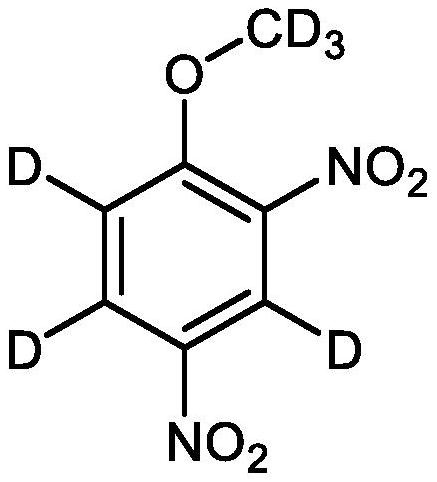
Perdeuterated 2, 4-dinitroanisole and preparation method thereof
A technology of dinitroanisole and deuterated anisole, which is applied in the field of synthesis of deuterated energetic materials, can solve the problems that are unfavorable to industrial operation, unfavorable to industrial production, long reaction cycle, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A pressure vessel with a volume of 50 mL and filled with 6.5 g of deuterated acetic anhydride and 51 g of carbon tetrachloride was placed in a high-precision medium-temperature circulating bath at -5 °C, and was poured through a constant-pressure dropping funnel at a rate of 0.5 mL / min. Add deuterated nitric acid with a concentration of 98% in the container dropwise, and mechanically stir at a rate of 150 rpm while adding drops, and keep the temperature of the mixed solution not higher than 5°C. After 28 minutes, the dropwise addition is completed, and the temperature of the mixed solution is raised to 30°C. ℃; slowly add 2.3g of deuterated anisole into the mixed solution in 4 batches, maintain the temperature of the mixed solution at 30℃±0.5℃ during the addition process, then add 0.23g of sodium nitrate, after the addition is completed, seal the reaction device and increase Stirring rate to 400rpm, heat preservation for 4 hours, pour the mixture into 10mL deionized wate...
Embodiment 2
[0026] Place a pressure-resistant container with a volume of 100 mL and filled with 20.5 g of deuterated acetic anhydride and 175 g of carbon tetrachloride in a high-precision medium-temperature circulating bath at -5°C, and inject the Add deuterated nitric acid with a concentration of 95% in the medium dropwise, and mechanically stir at a rate of 150 rpm while adding drops, and keep the temperature of the mixed solution not higher than 5°C. After 51 minutes, the dropwise addition is completed, and the temperature of the mixed solution is raised to 25°C ; Slowly add 8.4g of deuterated anisole into the mixed solution in 6 batches, maintain the temperature of the mixed solution at 25°C ± 0.5°C during the addition process, then add 0.84g of potassium nitrate, after the addition, seal the reaction device and increase the stirring Speed up to 400rpm, keep warm for 4 hours, pour the mixture into 25mL of deionized water at 5°C, stir at 50rpm for 1 hour, filter with suction, wash the...
Embodiment 3
[0029] Place a pressure-resistant container with a volume of 100 mL and filled with 23.5 g of deuterated propionic anhydride and 190 g of carbon tetrachloride in a high-precision medium-temperature circulating bath at -5°C, and inject the Add deuterated nitric acid with a concentration of 98% in the medium dropwise, and mechanically stir at a rate of 200 rpm while adding drops, and keep the temperature of the mixed solution not higher than 5°C. After 36 minutes, the dropwise addition is completed, and the temperature of the mixed solution is raised to 25°C ;Add 6.5g of deuterated anisole slowly to the mixed solution in 5 batches, maintain the temperature of the mixed solution at 25°C±0.5°C during the addition process, then add 0.654g of nickel nitrate, after the addition, seal the reaction device and increase the stirring Speed up to 400rpm, keep warm for 5 hours, pour the mixture into 20mL of deionized water at 5°C, stir at 50rpm for 0.5 hours, filter with suction, wash the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Crystal density | aaaaa | aaaaa |
| Crystal density | aaaaa | aaaaa |
| Hot | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com