H2 receptor blocking drug efficacy related gene SLC22A1 polymorphism detection kit
A technology of polymorphism detection and receptor blocking, applied in the biological field, achieves short time-consuming, convenient use, and strong specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] A primer composition for detecting the SLC22A1 polymorphism of an H2 receptor blocking drug efficacy-related gene, including a C1022T detection primer and an internal control gene detection primer, the sequences of which are shown in Table 1 and Table 2 below.
[0054] Table 1 Sequences of detection primers for SLC22A1 gene polymorphisms (ie, C1022T detection primers)
[0055] primer name
Base sequence (5′→3′ direction)
P1-FW1 (C1022T detection primer 1)
5′-GCAGACCTGTTCCGCACGTC-3
P1-FW2 (C1022T detection primer 2)
5′-CAGACCTG TTCCGCACGAT-3′
P1-R (C1022T detection primer three)
5′-CACTCACAACTATATTACA-3′
[0056] Table 2 Sequences of primers for internal control gene detection
[0057] primer name
Embodiment 2
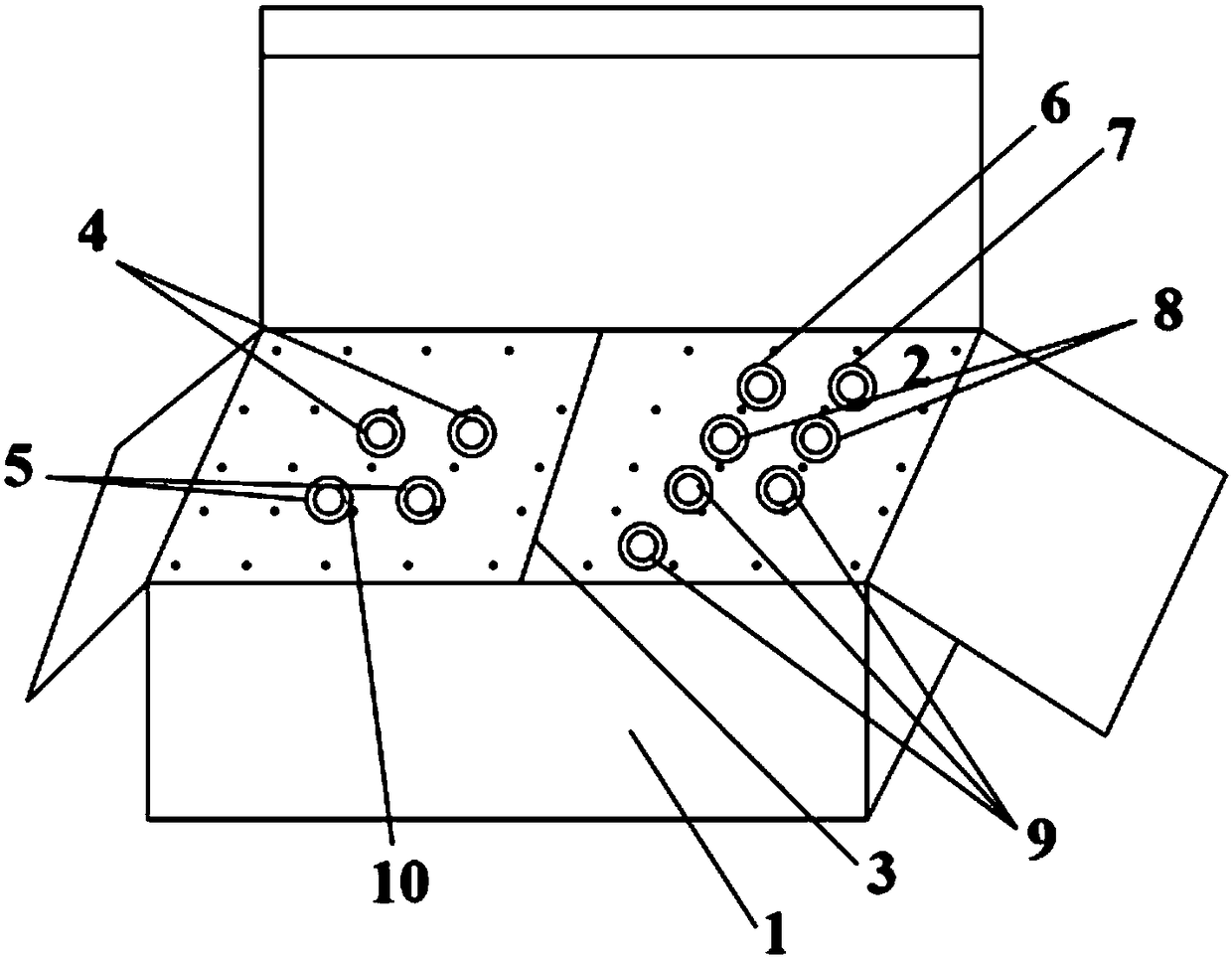
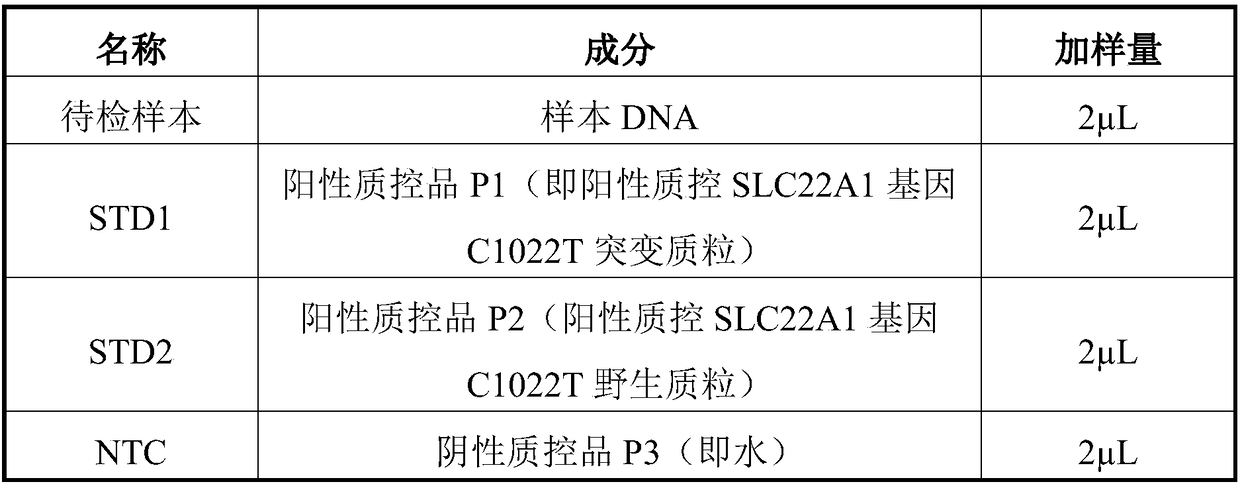
[0059] like figure 1 As shown, the kit containing the primer composition as described in Example 1 includes: a box body 1 with a box lid, a baffle plate 3, two sponges 2, two test tubes containing distilled water 4, two containing PCR Test tube for reaction master mix 5, one test tube containing positive quality control SLC22A1 gene C1022T mutant plasmid 6, one test tube containing positive quality control SLC22A1 gene C1022T wild plasmid 7, two test tubes containing internal control gene detection primers 8, three test tubes Tube 9 with primers for SLC22A1 gene detection. Wherein, the baffle 3 is placed in the box body 1, the box body 1 is divided into two parts, the two sponges 2 are respectively placed in the box body 1 on both sides of the baffle plate 3, the sponge 2 is punched with container holes 10, and the two One test tube containing distilled water 4, two test tubes containing PCR reaction premix 5 placed in the container hole 10 of sponge 2 on one side of baffle 3...
Embodiment 3
[0062] The operation method of the test kit of above-mentioned embodiment 2 is:
[0063] (1) Sample processing and nucleic acid extraction
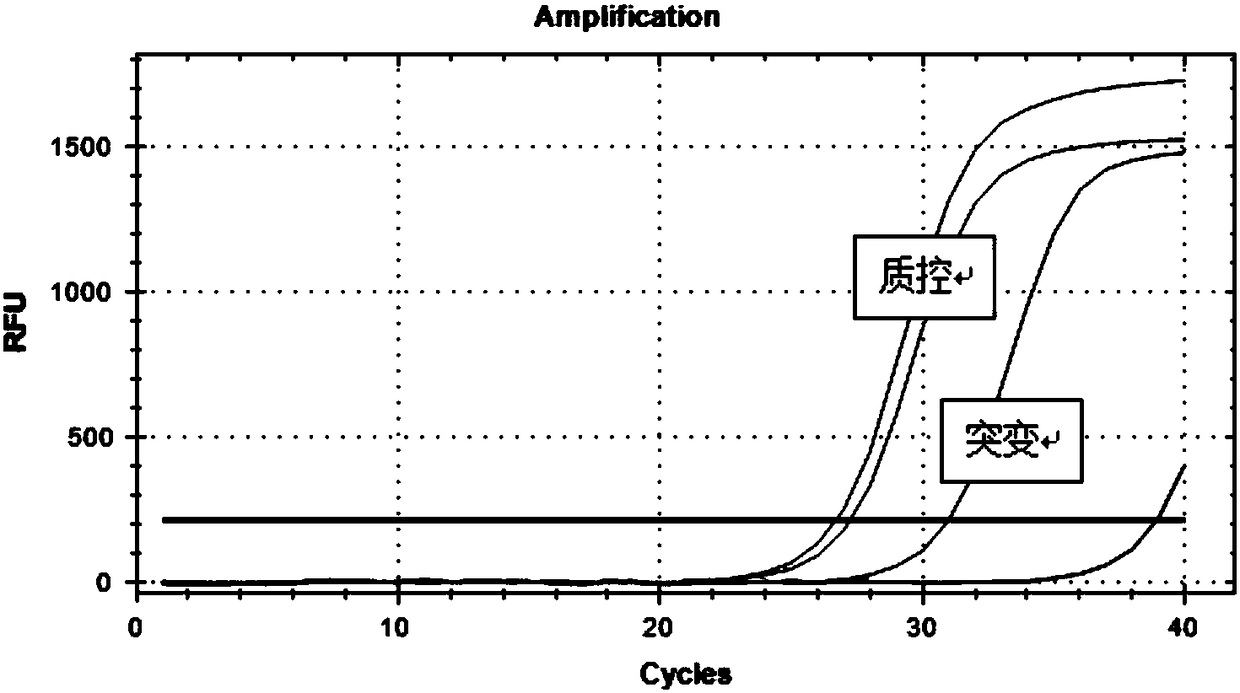
[0064] The samples were treated with commercial DNA extraction kits, and the concentration and purity of the extracted DNA should be determined with a UV spectrophotometer. 260 / OD 280 The value should be between 1.8 and 2.0, and the concentration should be between 5 and 50 ng / μL. Those with unqualified DNA quality should not be used for detection. It is recommended to re-extract nucleic acid if the DNA quality is lower than 5 ng / μL, and appropriate if it is higher than 50 ng / μL. Dilute to the specified concentration range. It is recommended to test the extracted DNA immediately. Otherwise, please store it below -20°C for no longer than 6 months. The kit quality control materials (ie, various plasmids) were thawed at room temperature before use, vortexed for 10 seconds, and centrifuged at 2000 rpm for 15 seconds before use.
[0065] (2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com