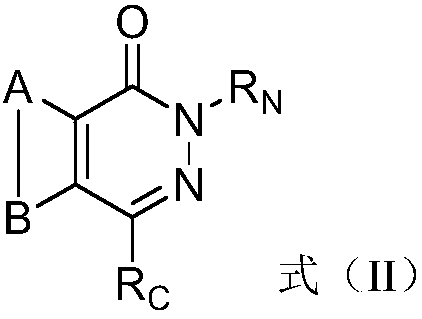
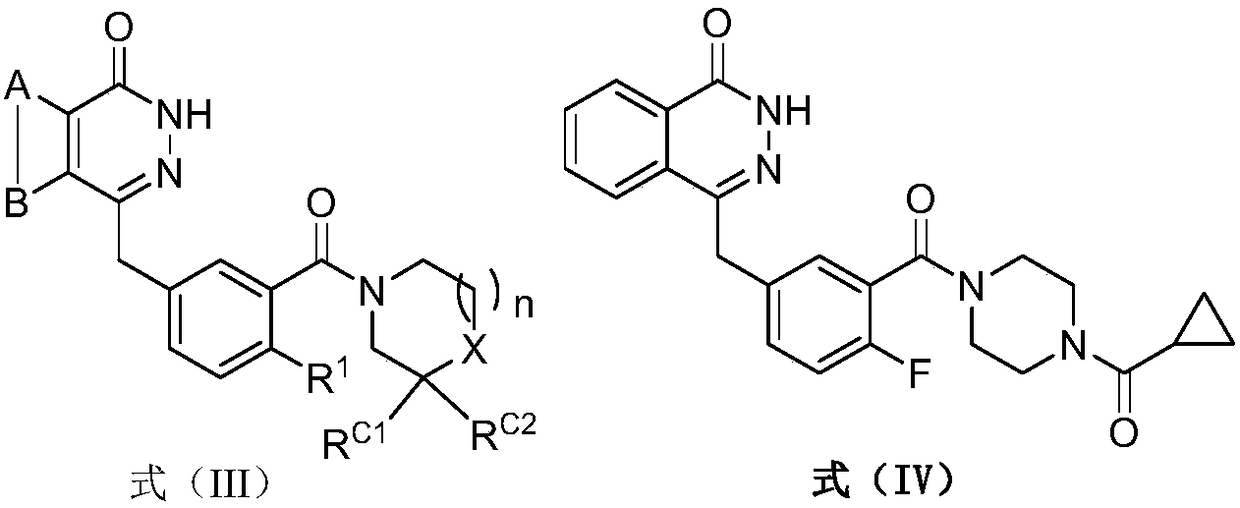
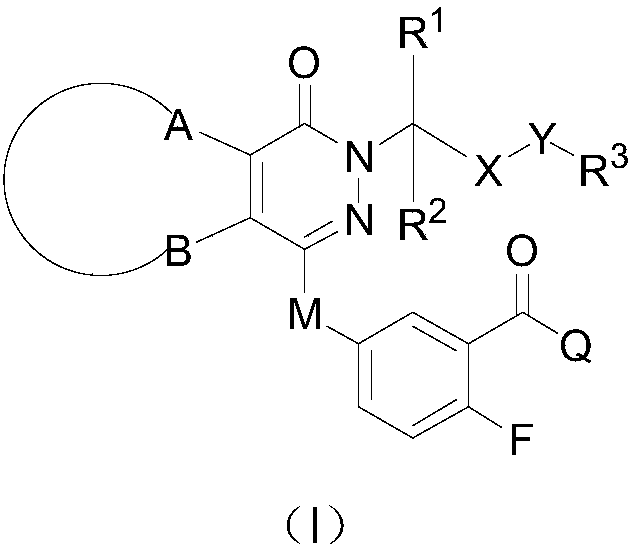
Pyridazinone derivative prodrugs or pharmaceutically acceptable salt thereof as well as pharmaceutical composition and application of prodrugs
A derivative, the technology of phthalazinone, applied in the field of medicinal chemistry, can solve the problems of poor solubility and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: Synthesis of 4-(4-fluoro-3-(1-acryloylpiperazin-4-yl-carbonyl)-benzyl)phthalazin-1(2H)ketone (compound 7)
[0072] Scheme 1:
[0073]
[0074] Add 4-(4-fluoro-3-(piperazin-1-yl-carbonyl)-benzyl)phthalazin-1(2H)ketone (5) (0.78g, 2.13mmol) into a 25ml three-necked flask, and Add dichloromethane (6.5ml) and triethylamine (0.52g, 5.14mmol), stir to dissolve, cool down to 1-10°C, add acryloyl chloride (230mg, 2.56mmol) dropwise, raise to room temperature after dropwise, stir 1h. TLC showed that the reaction was complete; the reaction solution was directly concentrated to dryness, the residue was slurried with water, stirred for 1 h and then filtered to obtain an off-white solid, which was purified by a silica gel column to obtain a white solid product (330 mg), with a yield of 37%. 1 HNNR (400MHz, CDCl 3 )δ:10.98(1H,bs),8.49(1H,s),7.80-7.75(3H,m),7.36(2H,d,J=4.4Hz),7.06(1H,t,J=8.8Hz), 6.59-6.52(1H,m),6.34(1H,d,J=16.4),5.76(1H,m),4.32(2H,s),3.82-3.35(8H,m)....
Embodiment 2
[0075] Example 2: Synthesis of 4-(4-fluoro-3-(1-(2-butenoyl)piperazin-4-yl-carbonyl)-benzyl)phthalazin-1(2H)ketone (Compound 9)
[0076] Scheme 2:
[0077]
[0078] Add 4-(4-fluoro-3-(piperazin-1-yl-carbonyl)-benzyl)phthalazin-1(2H)ketone (5) (0.78g, 2.13mmol) into a 25ml three-necked flask, and Add dichloromethane (6.5ml) and triethylamine (0.52g, 5.14mmol), stir to dissolve, cool down to 1-10°C, then add 2-butenyl chloride (268mg, 2.56mmol) dropwise, dropwise To room temperature, stirred for 1h. TLC showed that the reaction was complete; the reaction solution was directly concentrated to dryness, the residue was slurried with water, stirred for 1 hour and then filtered to obtain an off-white solid, which was purified by a silica gel column to obtain a white solid product
[0079] (450 mg), yield 37%. 1 HNNR (400MHz, CDCl 3 )δ:11.07(1H,bs),8.49(1H,m),7.81-7.73(3H,m),7.36-7.33(2H,m),7.06(1H,t,J=8.8Hz),6.96-6.90 (1H,m),6.26(1H,s),5.21(1H,m),4.31(2H,s),3.80-3.21(8H,m),1....
Embodiment 3
[0080] Example 3: 4-(4-fluoro-3-(1-(3-methyl-2-butenoyl)piperazin-4-yl-carbonyl)-benzyl)phthalazin-1(2H)ketone ( Compound 11) Synthesis
[0081] Scheme 3:
[0082]
[0083] Add 4-(4-fluoro-3-(piperazin-1-yl-carbonyl)-benzyl)phthalazin-1(2H)ketone (5) (0.78g, 2.13mmol) into a 25ml three-necked flask, and Add dichloromethane (6.5ml) and triethylamine (0.52g, 5.14mmol), stir to dissolve, cool down to 1-10°C, then add 3-methylcrotonyl chloride (304mg, 2.56mmol) dropwise, dropwise To room temperature, stirred for 1h. TLC showed that the reaction was complete; the reaction solution was directly concentrated to dryness, the residue was slurried with water, stirred for 1 hour and then filtered to obtain an off-white solid, which was purified by a silica gel column to obtain a white solid product
[0084] (600 mg), yield 63%. 1 HNNR (400MHz, CDCl 3 )δ:11.01(1H,m),8.49(1H,m),7.80-7.73(3H,m),7.34(2H,m),7.08-7.04(1H,m),5.77(1H,s),4.31 (2H,s),3.78-3.11(8H,m),1.92-1.79(6H,m).MS(ESI...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com