Preparation method of high-quantum-yield dual-emitting fluorescent carbon dots and application thereof in detection of PFOS (perfluorooctane sulfonates)
A dual-emission fluorescent and high-quantum technology, applied in the field of nanomaterials, can solve the problems of low quantum yield and difficulty in popularizing the application of fluorescent carbon quantum dots, and achieve the effect of simple preparation method, easy operation of preparation method, and cheap and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
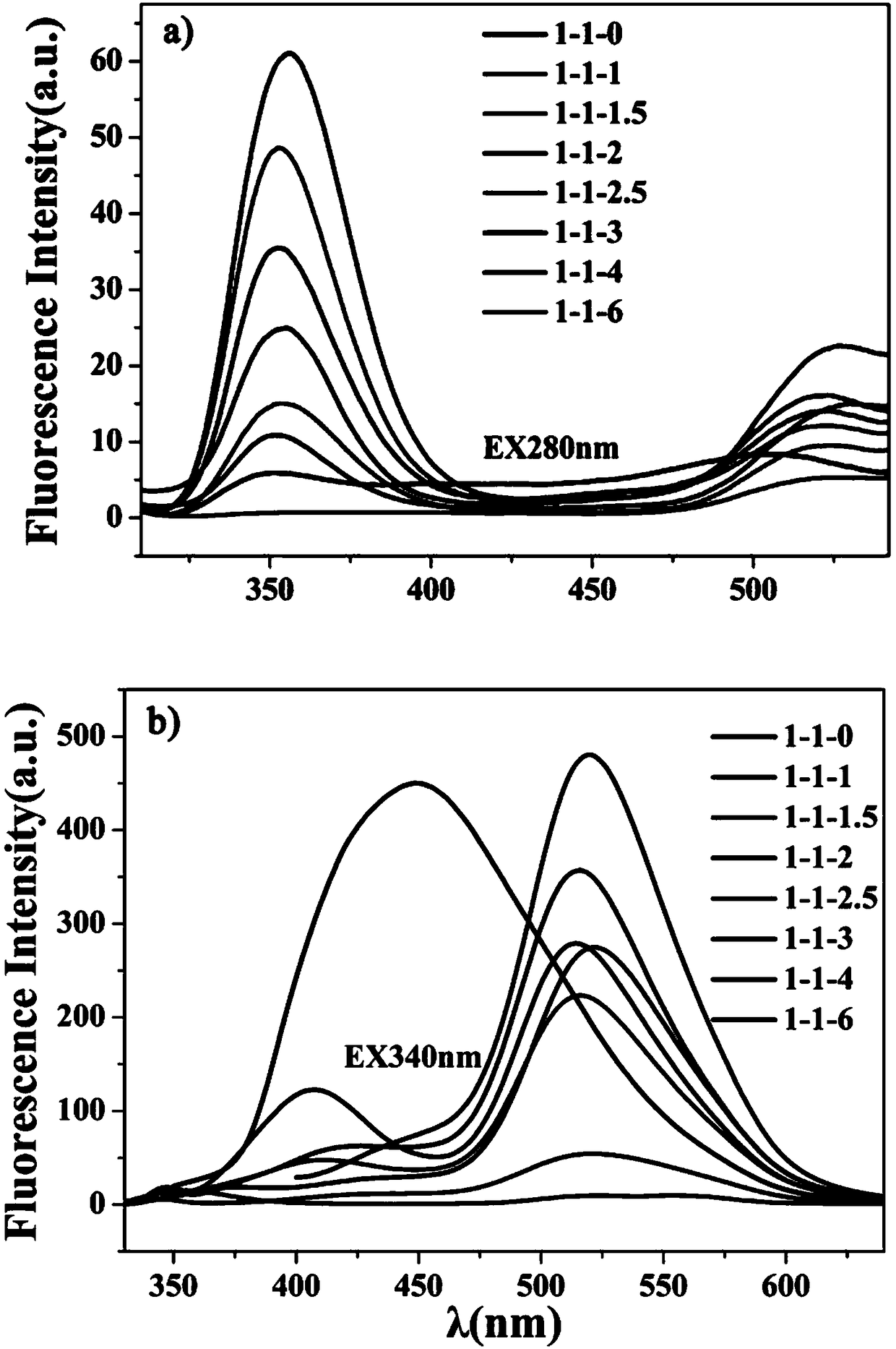
[0045] The influence of embodiment 1 ethylenediamine and phosphoric acid ratio on carbon dot
[0046]Accurately weigh eight parts of 3mmol 2,4-diaminotoluene and 200μL ethylenediamine in eight 25mL polytetrafluoroethylene reaction kettles, and then use volume ratio ethylenediamine:phosphoric acid=1:(0, 1, 1.5 , 2, 2.5, 3, 4, 6) Measure 0, 200 μL, 300 μL, 400 μL, 500 μL, 600 μL, 800 μL, and 1200 μL of phosphoric acid solution in corresponding reaction kettles respectively, and then add 4 mL of water to each of the eight reaction kettles. Then the closed reaction kettle was placed in a blast drying oven at 200° C. for 10 h. After the reaction is completed, the reaction kettle is naturally cooled to room temperature, and the product is centrifugally dialyzed and freeze-dried to obtain a carbon dot solid.
[0047] Dilute the obtained carbon dots with water to prepare a carbon dot mother solution with a concentration of 1 mg / mL, take 200 μL, dilute to 1 mL with water, and measure ...
Embodiment 2
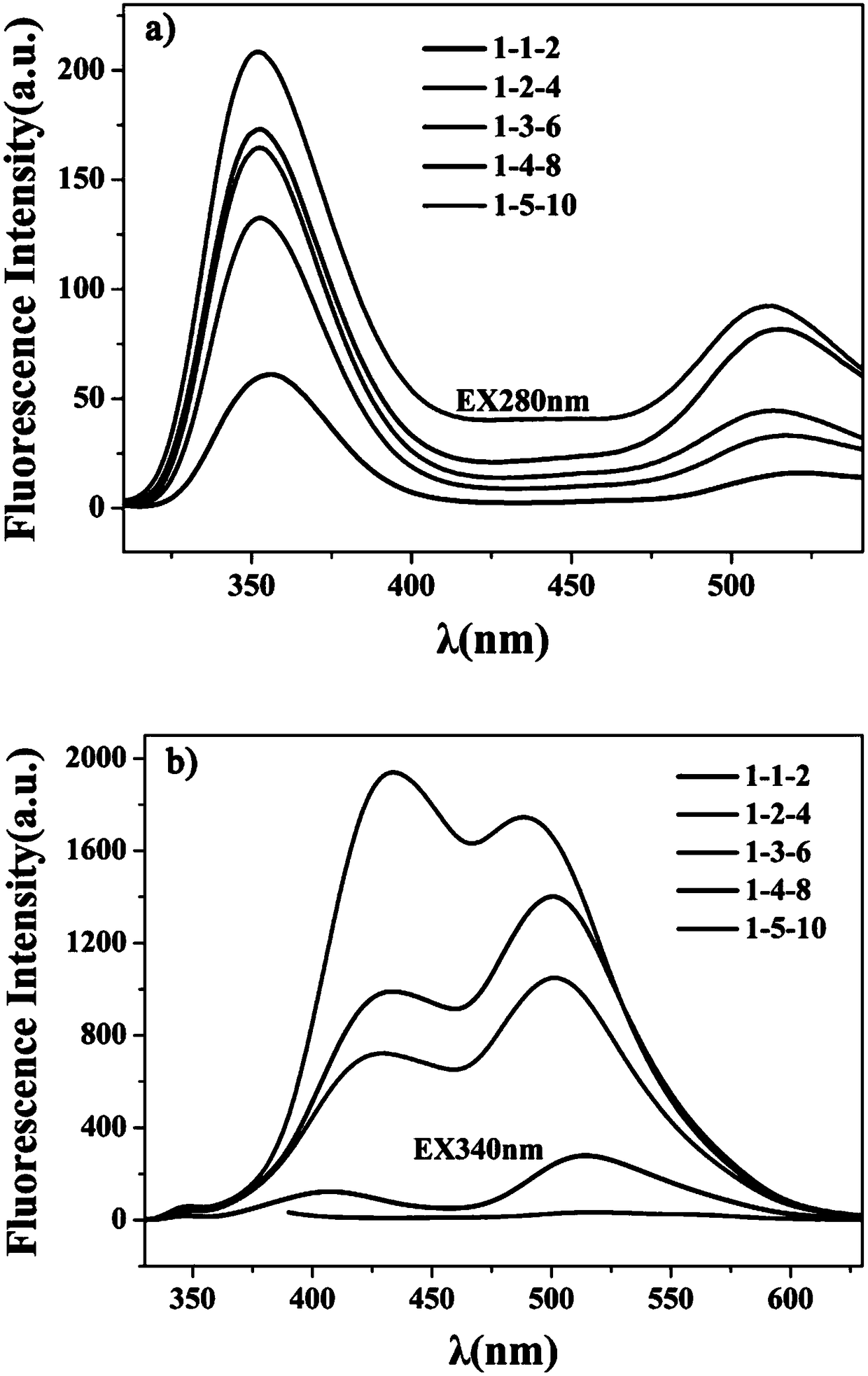
[0048] The influence of embodiment 2 ethylenediamine and phosphoric acid volume on carbon point
[0049] Accurately weigh five parts of 3mmol 2,4-diaminotoluene in five 25mL polytetrafluoroethylene reaction kettles, and then simultaneously expand the volume of the two according to the volume ratio of ethylenediamine:phosphoric acid=1:2 and add them to the reaction In the kettle, measure 200 μL, 400 μL, 600 μL, 800 μL, and 1000 μL of ethylenediamine, and the corresponding phosphoric acid volumes are 400 μL, 800 μL, 1200 μL, 1600 μL, and 2000 μL, respectively. Next, 4 mL of water was added to the reaction kettle, and then the closed reaction kettle was placed in a blast drying oven at 200° C. for 10 h of heating reaction. After the reaction is completed, the reaction kettle is naturally cooled to room temperature, and the product is centrifugally dialyzed and freeze-dried to obtain a carbon dot solid.
[0050] Adopt the method identical with embodiment 1 to obtain its fluoresce...
Embodiment 3
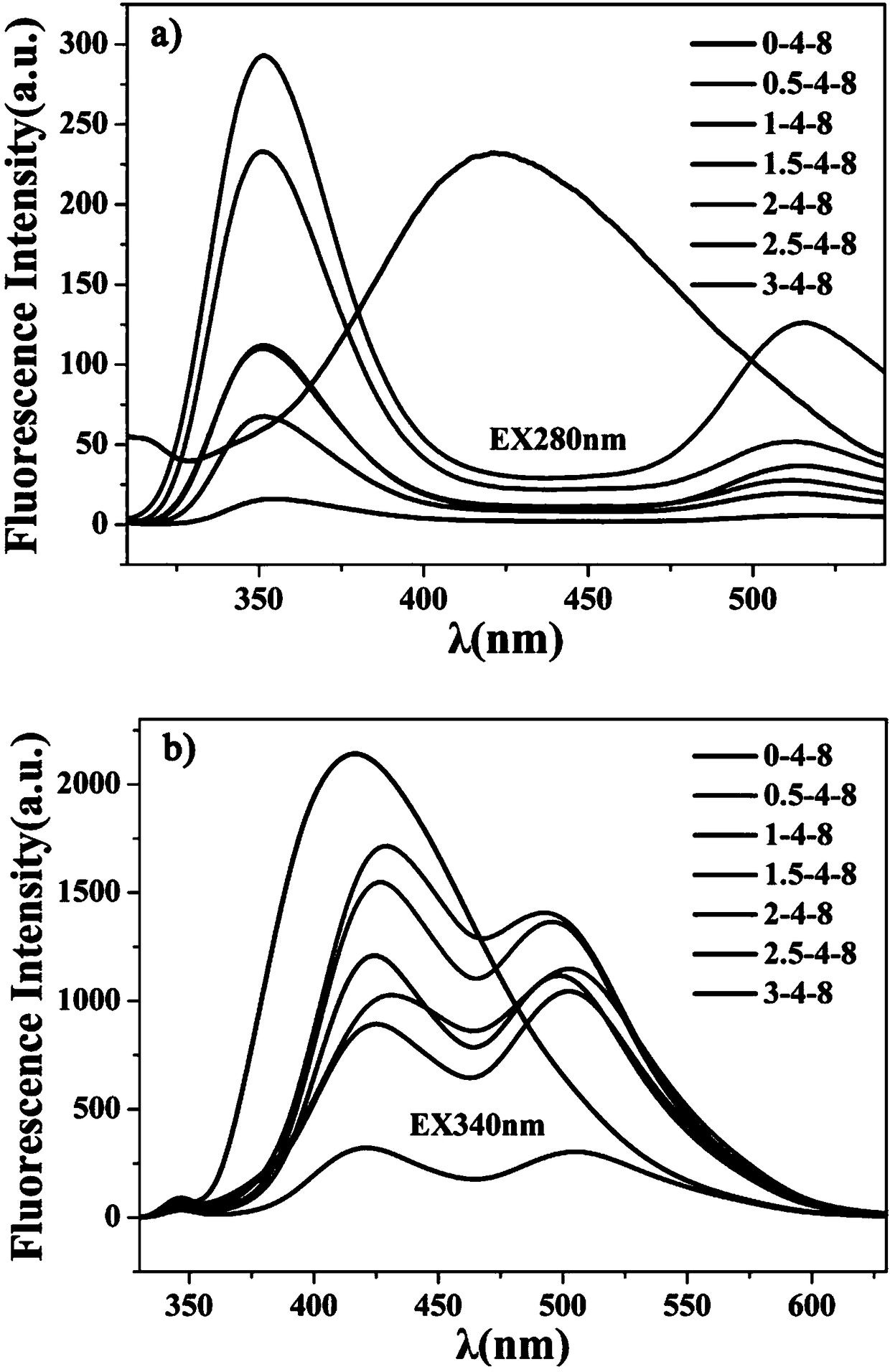
[0051] Example 3 Effect of 2,4-diaminotoluene on carbon dots
[0052] First weigh 0, 1.5mmol, 3mmol, 4.5mmol, 6mmol, 7.5mmol, 9mmol 2,4-diaminotoluene in the reaction kettle respectively, (the ratio of the amount of corresponding substances is 2,4-diaminotoluene:ethylene diaminotoluene Amine: phosphoric acid = 0:4:8, 0.5:4:8, 1:4:8, 1.5:4:8, 2:4:8, 2.5:4:8, 3:4:8) and then measure them accurately Take seven portions of 800 μL phosphoric acid and 1600 μL of ethylenediamine in seven 25 mL polytetrafluoroethylene reactors. Next, 4 mL of water was added to the reaction kettle, and then the closed reaction kettle was placed in a blast drying oven at 200° C. for 10 h of heating reaction. After the reaction is completed, the reaction kettle is naturally cooled to room temperature, and the product is centrifugally dialyzed and freeze-dried to obtain a carbon dot solid.
[0053] Adopt the method identical with embodiment 1 to obtain its fluorescence spectrogram ( image 3 a,b). Suc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com