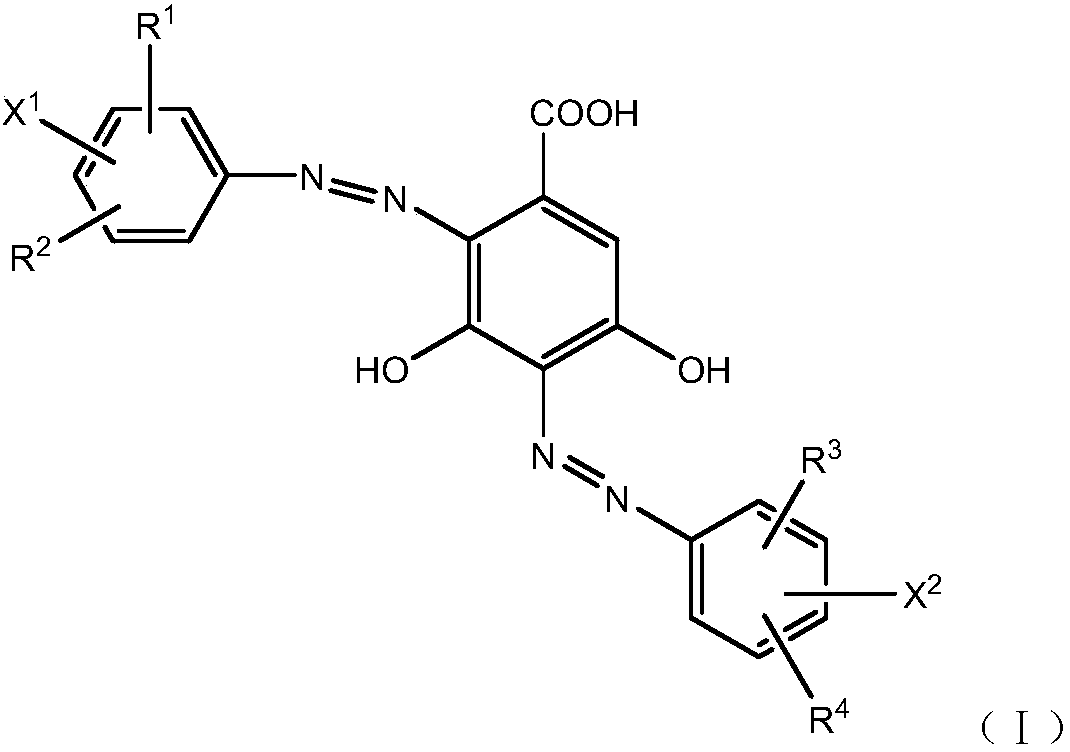
Orange reactive dye compound as well as preparation method and application thereof
A technology of reactive dyes and compounds, applied in the directions of reactive dyes, chemical instruments and methods, dyeing methods, etc., can solve the problems of broken bonds and hydrolysis, the fastness to washing cannot meet the production requirements, and the color fastness of dyed fabrics is deteriorated.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
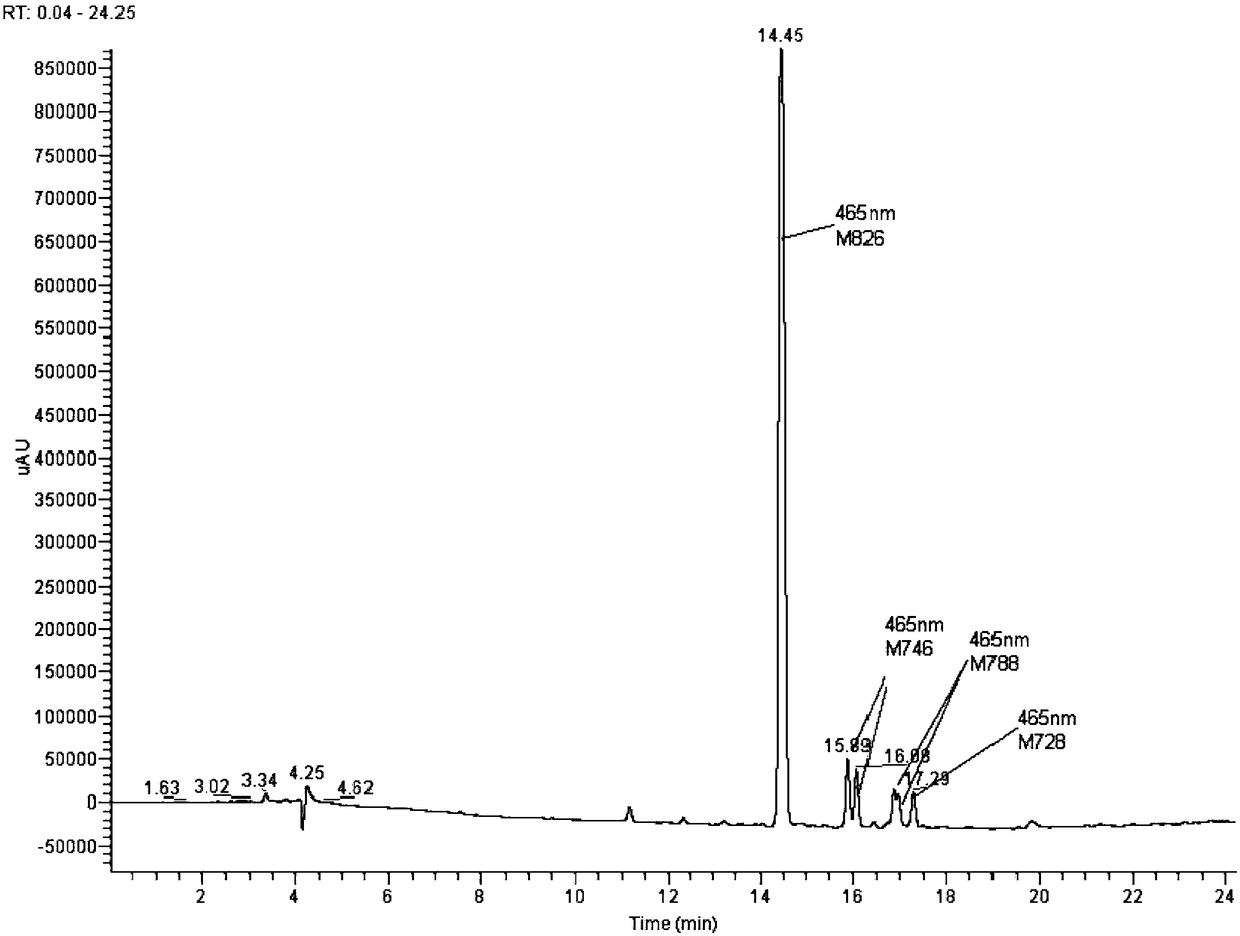
[0048] 1) Diazotization: Put 100g of water and 100g of ice into 32.5g (0.2mol) of crixidin para-ester (2-methoxy-5 methyl para-ester) for beating for 1 hour, then add 40g of 31% hydrochloric acid ( Containing HCl 0.34mol), add 48g 30% sodium nitrite solution (containing 0.208mol sodium nitrite) within 20-30min, control pH=0.5-2.0, temperature T=0-20°C to carry out diazotization reaction, use 4 - The ethanol solution of dimethylaminobenzaldehyde detects the end point (that is, no discoloration within 5s). After the diazo end point is reached, the excess sodium nitrite is eliminated with sulfamic acid, and the diazo solution is stored for later use.
[0049] 2) One-time coupling: add 15.4g (0.1mol) 3,5-dihydroxybenzoic acid into 200g water for beating, and after beating, add 0.1mol crixidin para-ester diazonium solution into 3,5-dihydroxybenzoic acid solution At the same time, use 30% liquid caustic soda to control the pH at 4.0, control the temperature at 0-20°C, and react for...
Embodiment 2~19
[0053] With reference to the preparation method of disazo dyes described in Example 1, the dye compounds of the structure shown in the following table 1 can be obtained respectively, the difference is that with sulfonated para-ester, para-ester, meta-ester, 2, 5-dimethoxy para-ester, 2-methoxy meta-ester, and other substituted aromatic amines respectively replace the Crixidin para-ester for diazo coupling:
[0054] Table 1
[0055]
[0056]
[0057]
[0058]
Embodiment 20~30
[0060] With reference to the preparation method of disazo dyes described in Example 1, the dye compounds of the structure shown in the following table 2 can be prepared respectively, the difference is that with sulfonated para-ester, para-ester, meta-ester, 2, 5-dimethoxy-para-ester, 2-methoxy-meta-ester, 2-methoxy-para-ester, 2-methyl-para-ester and other substituted aromatic amines respectively replaced the crixidin para-ester for heavy Nitrogen coupling. After the coupling is completed, adjust the pH value to 10-11 with liquid caustic soda as usual and maintain it at 25-30°C for 45-60 minutes, then add acid to adjust the pH value to 5-6.
[0061] Table 2
[0062]
[0063]
[0064]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com