Porphyrin sensitizer and synthesis method thereof
A sensitizer and porphyrin technology, applied in the field of porphyrin sensitizer solar cell sensitizer and its synthesis, can solve the problems of short life, low photoelectric conversion efficiency, poor stability, etc., and achieve spectral performance and thermal stability. Good, extensive research and application, the effect of good spectral responsiveness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
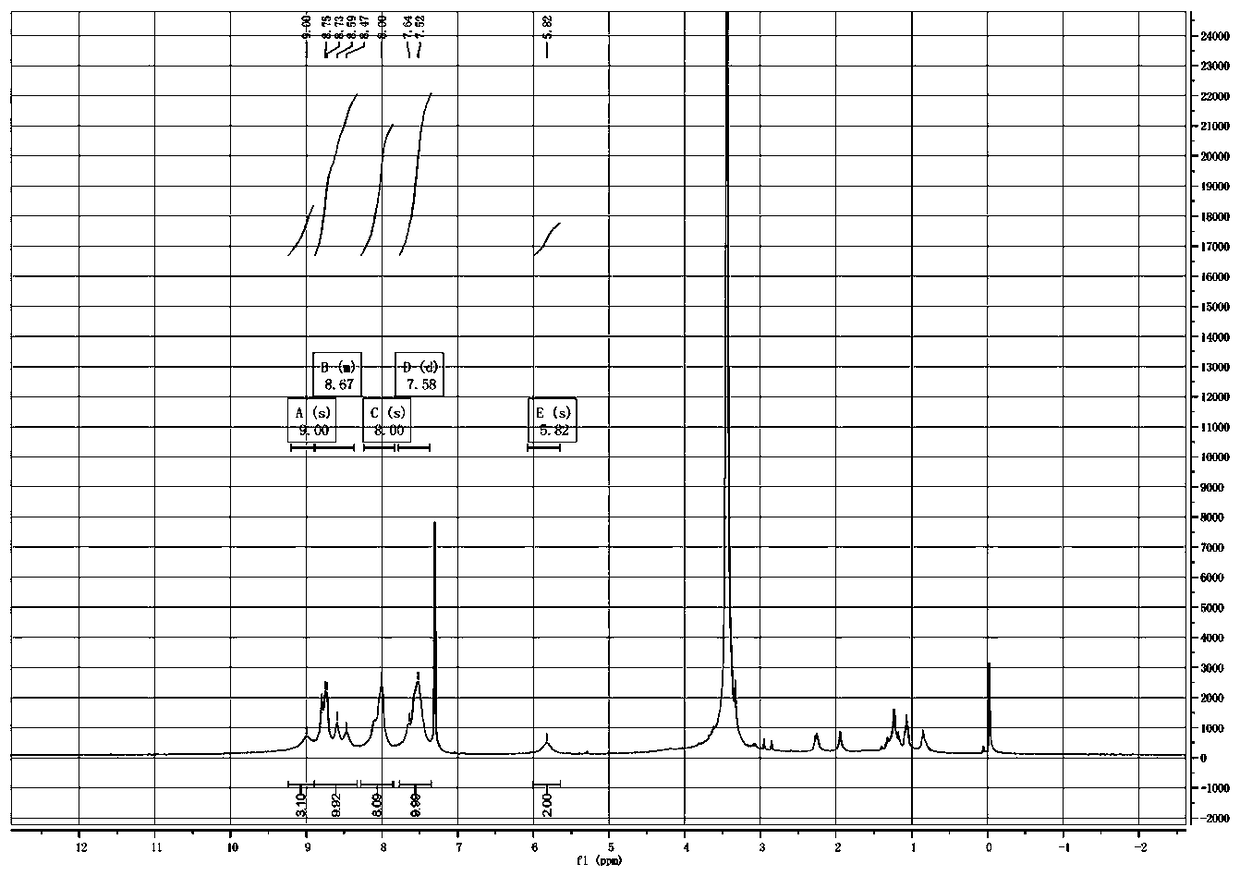
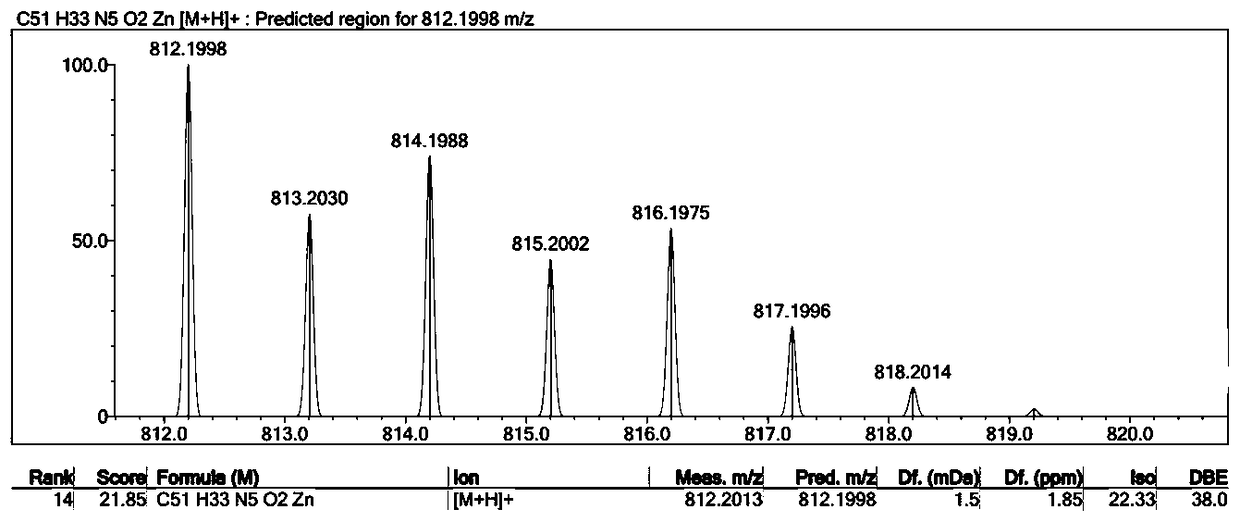
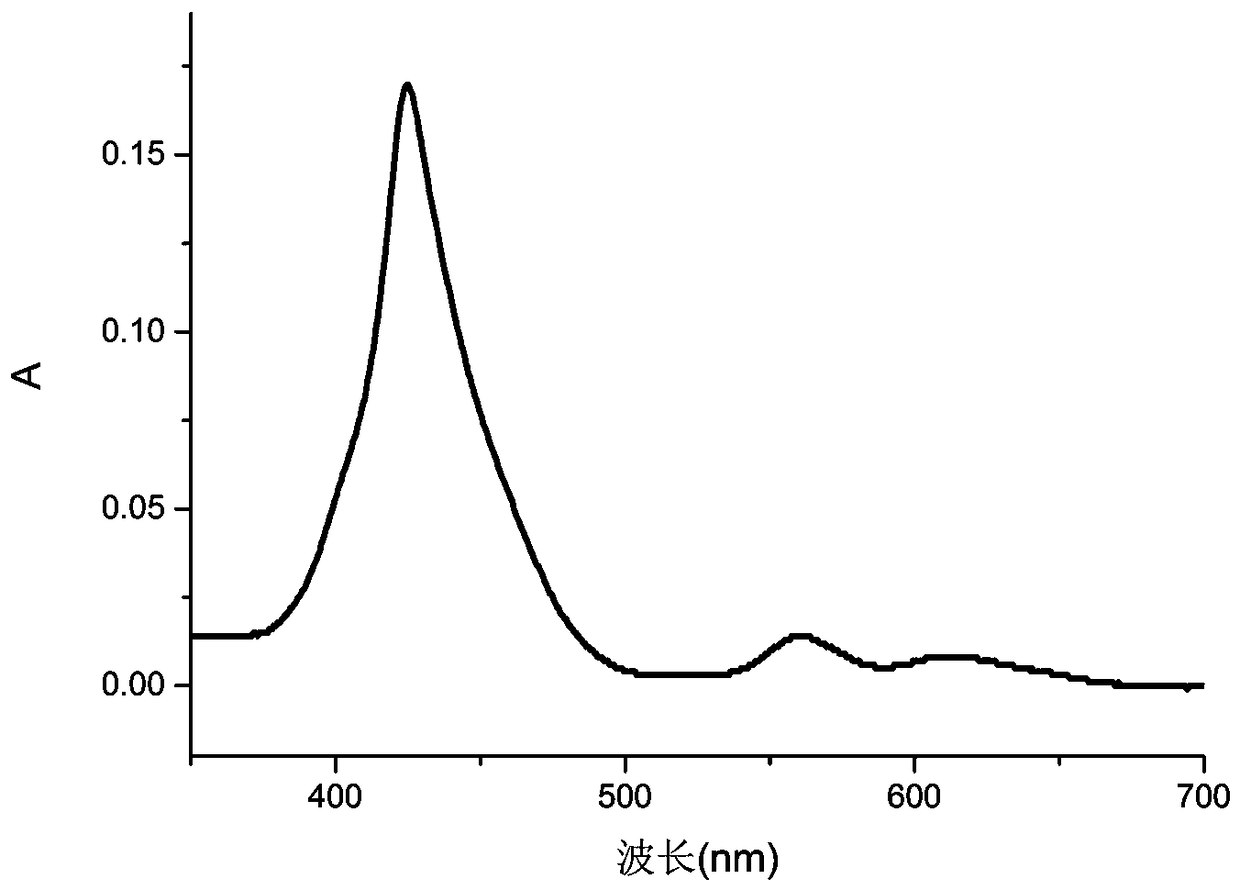
[0029] 1.59g (1.75mmol) of compound II (R substituting group is hydrogen) was dissolved in a mixed solvent of tetrahydrofuran and methanol (60ml, 1:1), and 10% lithium hydroxide aqueous solution (42mg, 1.75mmol) was added dropwise thereto , heated to reflux for 3h. Then it was neutralized to pH=6 with 0.2% hydrochloric acid, extracted with dichloromethane, concentrated, purified on a silica gel column, and recrystallized from dichloromethane and methanol (1:2) to obtain compound I with a yield of 80%.
[0030]
Embodiment 2
[0032] 1.59g (1.75mmol) of compound II (R substituent is hydrogen) was dissolved in a mixed solvent of tetrahydrofuran and methanol (60ml, 1:1), and 10% aqueous sodium hydroxide solution (70mg, 1.75mmol) was added dropwise thereto , heated to reflux for 3h. Then it was neutralized to pH=6 with 0.2% hydrochloric acid, extracted with dichloromethane, concentrated, purified on a silica gel column, and recrystallized from dichloromethane and methanol (1:2) to obtain compound I with a yield of 82%.
[0033]
Embodiment 3
[0035] 1.59g (1.75mmol) of compound II (R substituent is hydrogen) was dissolved in a mixed solvent of tetrahydrofuran and methanol (60ml, 1:1), and 10% potassium hydroxide aqueous solution (98mg, 1.75mmol) was added dropwise thereto , heated to reflux for 3h. Then it was neutralized to pH=6 with 0.2% hydrochloric acid, extracted with dichloromethane, concentrated, purified on a silica gel column, and recrystallized from dichloromethane and methanol (1:2) to obtain compound I with a yield of 85%.
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com