Probe for bacteria resistant to extended-spectrum beta-lactam and cephalosporin antibiotics as well as synthesis method and application of probe
A technology of cephalosporins and lactams, which is applied in the field of compounds, can solve the problems of indistinguishable decomposition, etc., and achieve the effect of simple and cheap operation, rapid detection and imaging
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0062] The preparation method of the above-mentioned probe compound, the steps are as follows:
[0063]
[0064] Under the protection of argon, add 1eq Pd(OAc) to the eggplant type reaction flask in sequence 2 , and 1eq P(o-Tol) 3 After repeated freezing and thawing for 3 times, stir at room temperature for 1 h to activate the catalyst; then add 2.5 eq cefdinir, 10 eq charge-absorbing halogenated compound, DMF and triethylamine (10:1) in sequence, perform 3 freezing and degassing operations, and finally The temperature of the reaction was raised to 80°C for 12 hours; to terminate the reaction, add 100 mL of ethyl acetate to dilute, then use 100 mL of 0.2 mol / L HCl solution, 100 mL of NaHCO 3 Saturated aqueous solution and 100mL saturated brine, anhydrous MgSO 4 Drying; concentrating the organic phase and purifying through a silica gel column, wherein the volume ratio of dichloromethane:methanol in the silica gel column is 5:1 to obtain the probe compound.
[0065] The ap...
Embodiment 1
[0076]
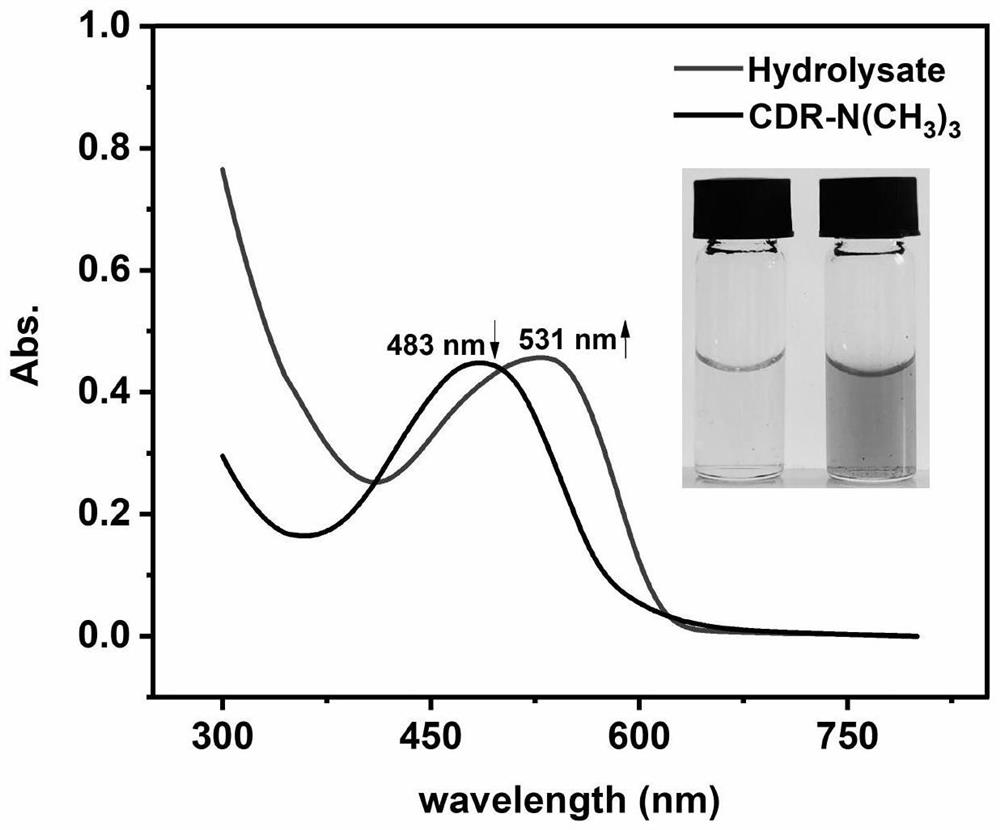
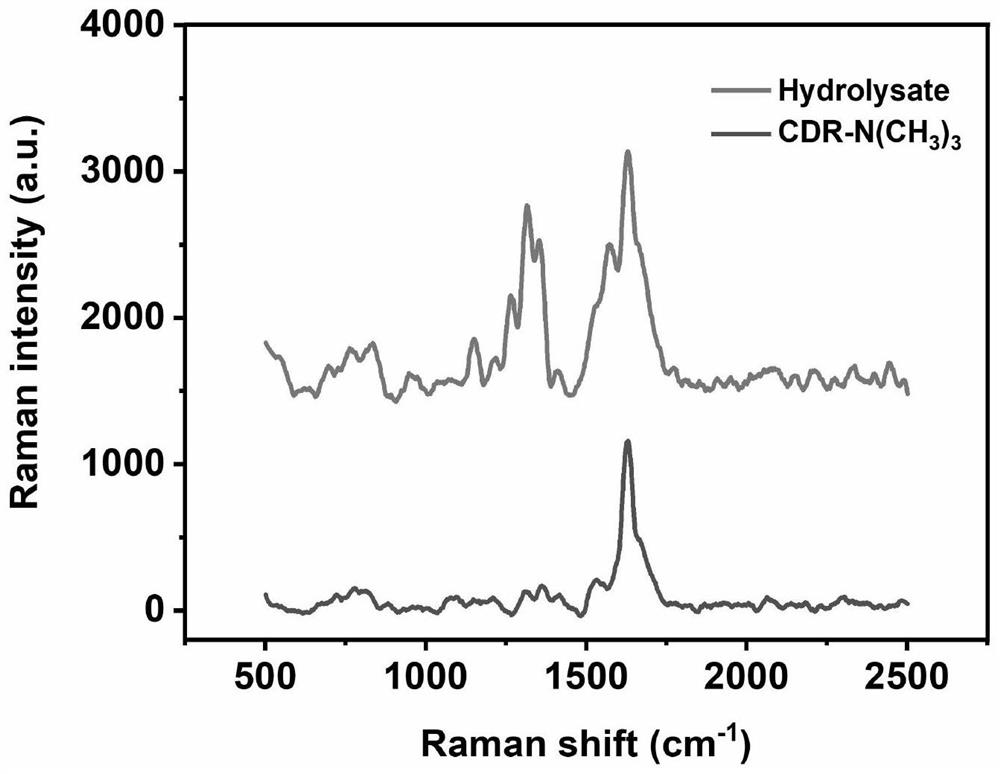
[0077] The above reaction formula is the structural change of the probe before and after enzymatic hydrolysis.
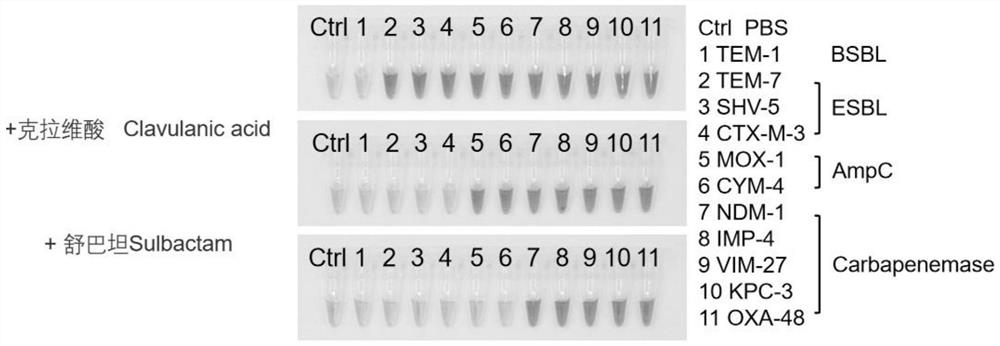
[0078] The result is as figure 1 and figure 2 shown, from figure 1 , figure 2 It can be seen that the probe amide ring can be hydrolyzed by extended-spectrum β-lactamase (ESBL) and cephalosporinase (AmpC) enzymes.
[0079]
[0080] Under argon protection, 4-bromo-N,N-dimethylaniline (1g, 5mmol) and methyl bromide (1.5g, 15mmol) were added to a single-necked flask containing 20mL ETOH, stirred at room temperature for 12h, and then heated to reflux 1h, until a large amount of light yellow solid is produced. Part of ETOH was rotary evaporated, recrystallized after refrigeration at -20°C, and the obtained precipitate was washed with pure water and ether in turn to obtain white solid compound 1 (1.02 g, yield 95%). Characterization data of the compound: 1H NMR (400MHz, MeOD) δ7.97 (d, J = 8Hz, 2H), 7.88 (d, J = 16Hz, 2H), 3.65 (s, 9H).13C NMR (101MH...
Embodiment 2
[0084]
[0085] Under the protection of argon, add Pd(OAc) to the eggplant-type reaction flask in sequence 2 (446.66mg, 2mmol) and P(o-Tol) 3 (608.89mg, 2mmol), repeated freezing and thawing three times, then stirred at room temperature for 1 hour to activate the catalyst. Then add cefdinir (1.98g, 5mmol), 2,4-dinitroiodobenzene (5.88g, 20mmol), 30mL DMF and 3mL triethylamine in sequence, perform 3 freezing and degassing operations, and finally raise the temperature of the reaction to 80°C Reaction 12h. Terminate the reaction, add 100mL ethyl acetate to dilute, and then use 100mL0.2mol / L HCl solution, 100mL NaHCO 3 Saturated aqueous solution and 100mL saturated brine, anhydrous MgSO 4 dry. The organic phase was concentrated and purified by a silica gel column (dichloromethane:methanol=5:1) to obtain compound 3 (0.87 g, 31%) as a brown-yellow solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com