Methods for improving oxidation resistance of Mg(BH4)2 and regulating hydrolysis hydrogen production rate of Mg(BH4)2
A technology of magnesium borohydride and hydrogen production by hydrolysis, applied in chemical instruments and methods, borane/diborane hydride, hydrogen production, etc., can solve the problems of uncontrollable reaction rate, poor oxidation resistance, violent reaction, etc. , to achieve the effect of improving antioxidant capacity, strong antioxidant capacity and high activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
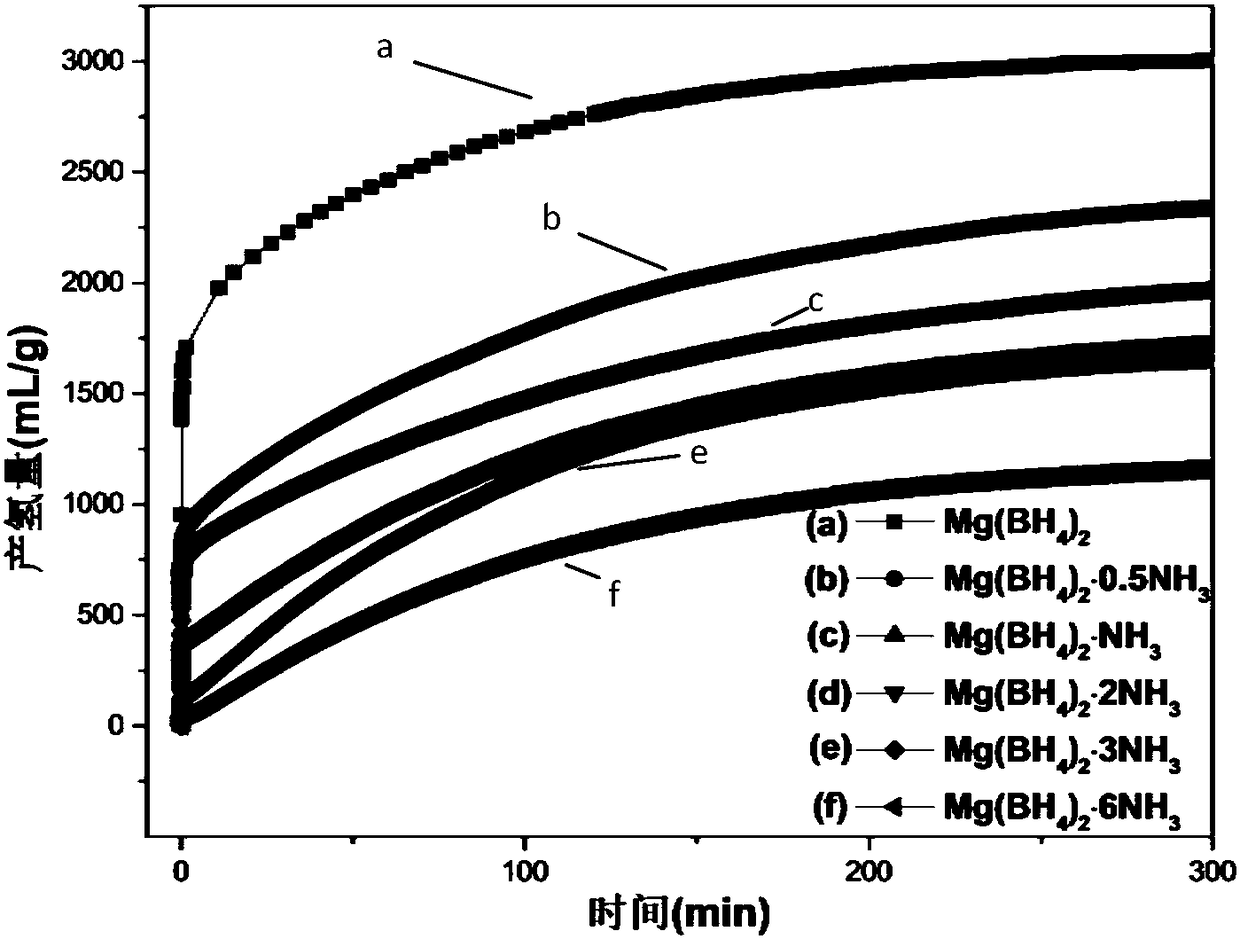
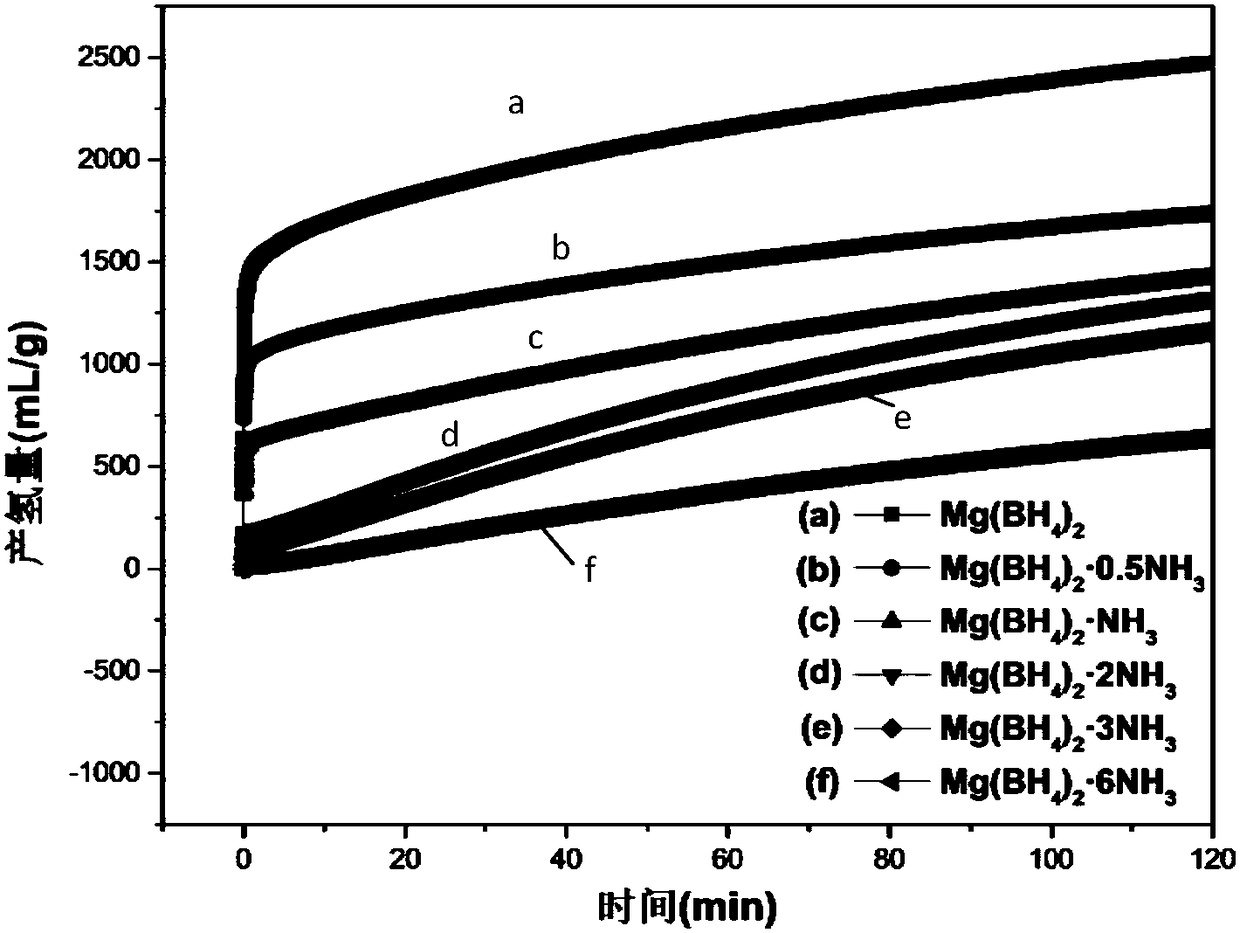
[0039] In a glove box with an argon atmosphere, take 0.05 g of Mg(BH 4 ) 2 Put it into a flask, and carry out hydrolysis reaction with 10mL pure water at room temperature, the reaction is mild, and the hydrolysis kinetic curve is as follows: figure 1 As shown in (a), 1g of the powder can release 2465mL of hydrogen gas within 1h, and finally can release a total of 3005mL of hydrogen gas. It can be seen that Mg(BH 4 ) 2 It has a very high hydrolysis hydrogen release rate and hydrogen release capacity.
Embodiment 2
[0041] In a glove box with argon atmosphere, take 0.05gMg(BH 4 ) 2 0.5NH 3 Put it into a flask, and carry out hydrolysis reaction with 10mL pure water at room temperature, the reaction is mild, and the hydrolysis kinetic curve is as follows: figure 1 As shown in (b), 1g of the powder can release 1513mL of hydrogen within 1h, and finally a total of 2376mL of hydrogen can be released. Compared to Mg(BH 4 ) 2 For example, Mg(BH 4 ) 2 0.5NH 3 The hydrolysis reaction is more controllable and milder, the hydrolysis rate is slower, and still has a higher hydrogen production.
Embodiment 3
[0043] In a glove box with an argon atmosphere, take 0.05 g of Mg(BH 4 ) 2 ·NH 3 Put it into a flask, and carry out hydrolysis reaction with 10mL pure water at room temperature, the reaction is mild, and the hydrolysis kinetic curve is as follows: figure 1 As shown in (c), 1 g of the powder can release 1249 mL of hydrogen within 1 h, and finally a total of 2029 mL of hydrogen can be released. Compared to Mg(BH 4 ) 2 and Mg(BH 4 ) 2 0.5NH 3 , Mg(BH 4 ) 2 ·NH 3 The hydrolysis reaction is more moderate and controllable, the hydrolysis speed is slower, and a certain amount of hydrogen release is maintained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com