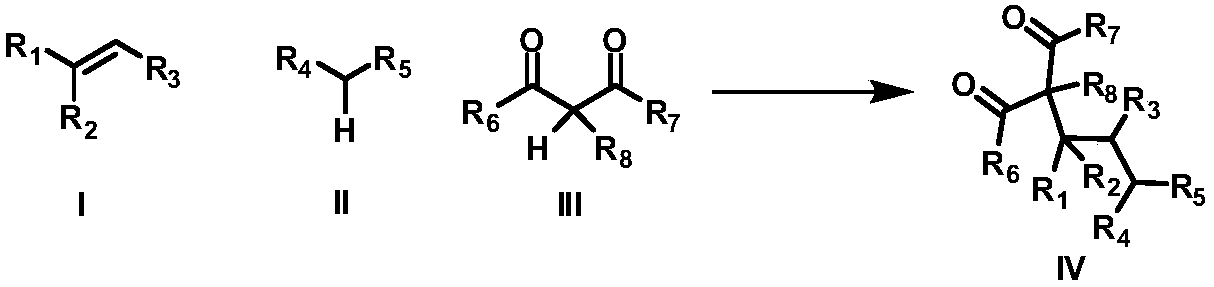
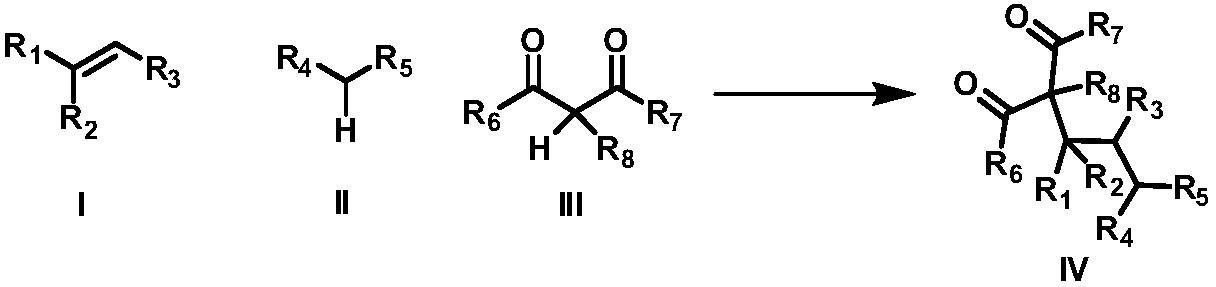
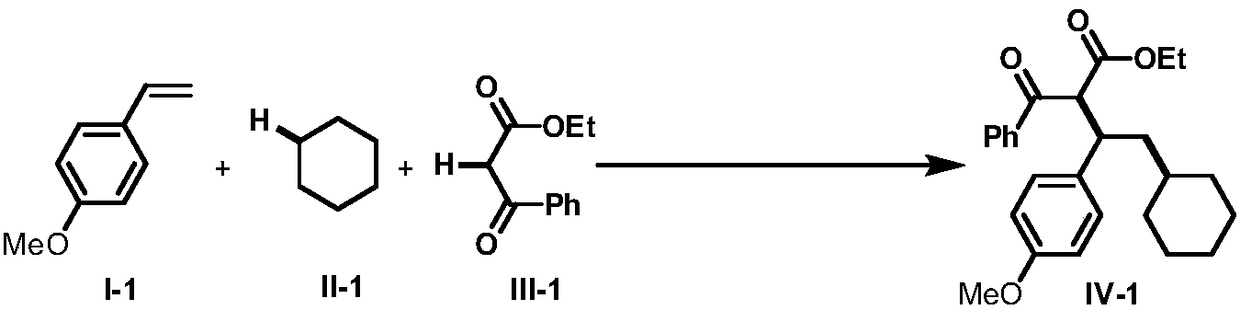
Intermolecular 1,2-dialkylation reaction method of olefine compound under photoredox/iron (II) catalytic system
A catalytic system and dialkylation technology, applied in the field of intermolecular 1,2-dialkylation reactions of olefinic compounds, can solve limited and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 17
[0065]
[0066] The p-methylstyrene of formula I-2 was used as the raw material, and the rest of the reaction raw materials, operations and parameters were the same as in Example 1 to obtain the target product IV-2 with a yield of 70%. dr=1.3:1; light yellow oily liquid; 1 H NMR (400MHz, CDCl 3)δ: 8.09(d, J=7.2Hz, 1.0H), 7.80(d, J=7.6Hz, 1.0H), 7.58(t, J=7.0Hz, 0.50H), 7.50-7.44(m, 1.66H ),7.33(t,J=6.8Hz,1.0H),7.17(d,J=7.6Hz,1.0H), 7.11-7.05(m,2.0H),6.94(d,J=7.6Hz,1H), 4.67-4.58(m,1.0H),4.23-4.15(m,1.0H),3.83-3.73(m,2.0H),2.32-2.20(m,4.0H),1.96-1.93(m,1.0H), 1.70-1.65(m, 1.0H), 1.54-1.43(m, 4.0H), 1.38-1.32(m, 1.0H), 1.26-1.20(m, 2.0H), 1.07-1.05(m, 2.0H), 0.94-0.84(m,4H); 13 C NMR (100MHz, CDCl 3 ( ), 41.5, 34.6, 34.4 (2C), 34.2, 31.7, 31.5, 26.5, 26.5, 26.1, 25.8, 21.1, 20.9, 14.1, 13.6; LRMS (EI, 70eV) m / z (%): 392 (M + ,1), 374(10), 278(28), 105(100); HRMS m / z(ESI) calcd for C 26 h 33 o 3 ([M+H] + ) 393.2424, found 393.2432.
Embodiment 18
[0068]
[0069] The p-bromostyrene of formula I-4 was used as the raw material, and the rest of the reaction raw materials, operations and parameters were the same as in Example 1 to obtain the target product IV-4 with a yield of 75%. dr=1.1:1; light yellow oily liquid; 1 H NMR (400MHz, CDCl 3 )δ: 8.08(d, J=7.6Hz, 2H), 7.61(t, J=7.2Hz, 1H), 7.50(t, J=7.6Hz, 2H), 7.43(d, J=8.0Hz, 2H) ,7.18(d,J=8.0Hz,2H),4.56(d,J=10.4Hz,1H), 3.82-3.76(m,3H),1.95-1.85(m,1H),1.72-1.61(m,2H ),1.54-1.34(m,7H), 1.07-1.02(s,2H),0.91-0.86(m,4H); 13 C NMR (100MHz, CDCl 3 )δ: 193.4, 167.7, 140.4, 136.7, 133.7, 131.4, 130.3, 128.8, 128.7, 120.5, 61.4, 61.3, 42.3, 42.0, 34.5, 34.4, 31.5, 26.4, 26.1, 25.8, 13.7, 70eVI )m / z(%):458(M + +2,1),456 (M + ,1),440(5),342(16),105(100);HRMS m / z(ESI)calcd for C 25 h 30 79 BrO 3 ([M+H] + ) 457.1373, found 457.1381.
Embodiment 19
[0071]
[0072] Using p-cyanostyrene of formula I-5 as raw material, the reaction temperature is 100° C., and the rest of the reaction raw materials, operations and parameters are the same as in Example 1 to obtain the target product IV-5 with a yield of 46%. dr=1.1:1; light yellow oily liquid; 1 H NMR (400MHz, CDCl 3 )δ:8.08(d,J=8.0Hz,1.0H),7.80(d,J=7.6Hz,1.0H), 7.63-7.60(m,1.6H),7.52-7.42(m,3.54H),7.41 -7.32(m,2.0H),4.66(d,J=10.8Hz,0.48H),4.62(d,J=10.8Hz,0.50H),4.22-4.15(m,1H),3.95-3.85(m, 1H), 3.80-3.75(m,1H),1.91-1.88(m,1.0H),1.71-1.34(m,6.0H),1.26-1.20(m,3H), 1.09-1.04(m,2H), 0.88-0.84(m,4H); 13 C NMR (100MHz, CDCl 3 )δ: 193.1, 192.8, 168.0, 167.4, 147.5, 147.3, 136.5, 136.3, 133.8, 133.5, 132.0, 129.4, 129.1, 128.8, 128.6, 128.5, 128.3, 118.7, 1178.7, 110.3.6, 6, ,60.8,42.8,42.51, 41.72,41.1,34.6,34.4,34.3,34.2,31.6,31.4,26.3,26.3,25.9,25.9,25.7,14.0,13.6; (EI,70eV)m / z(%):403 (M + ,1),298(11),192(8),105(100); HRMS m / z(ESI) calcd for C 26 h 30 NO 3 ([M+H] + ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com