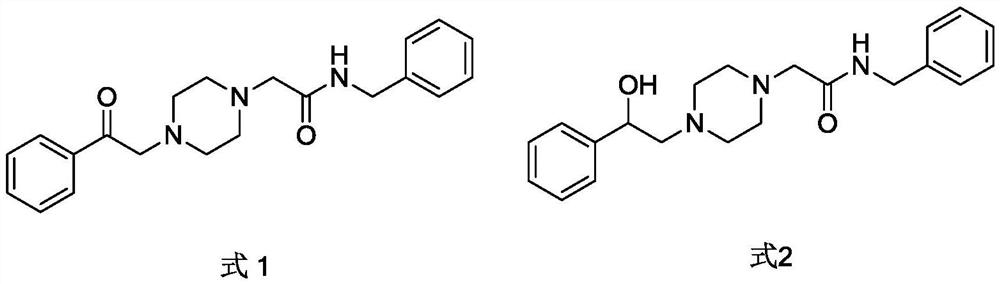
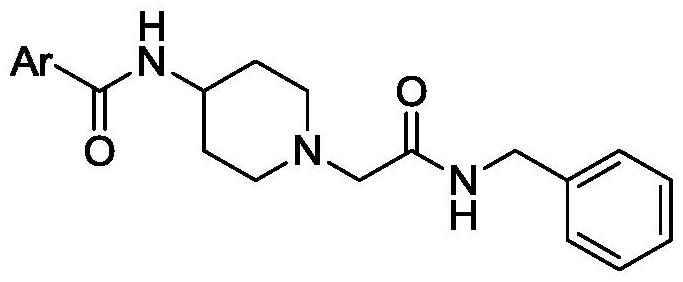
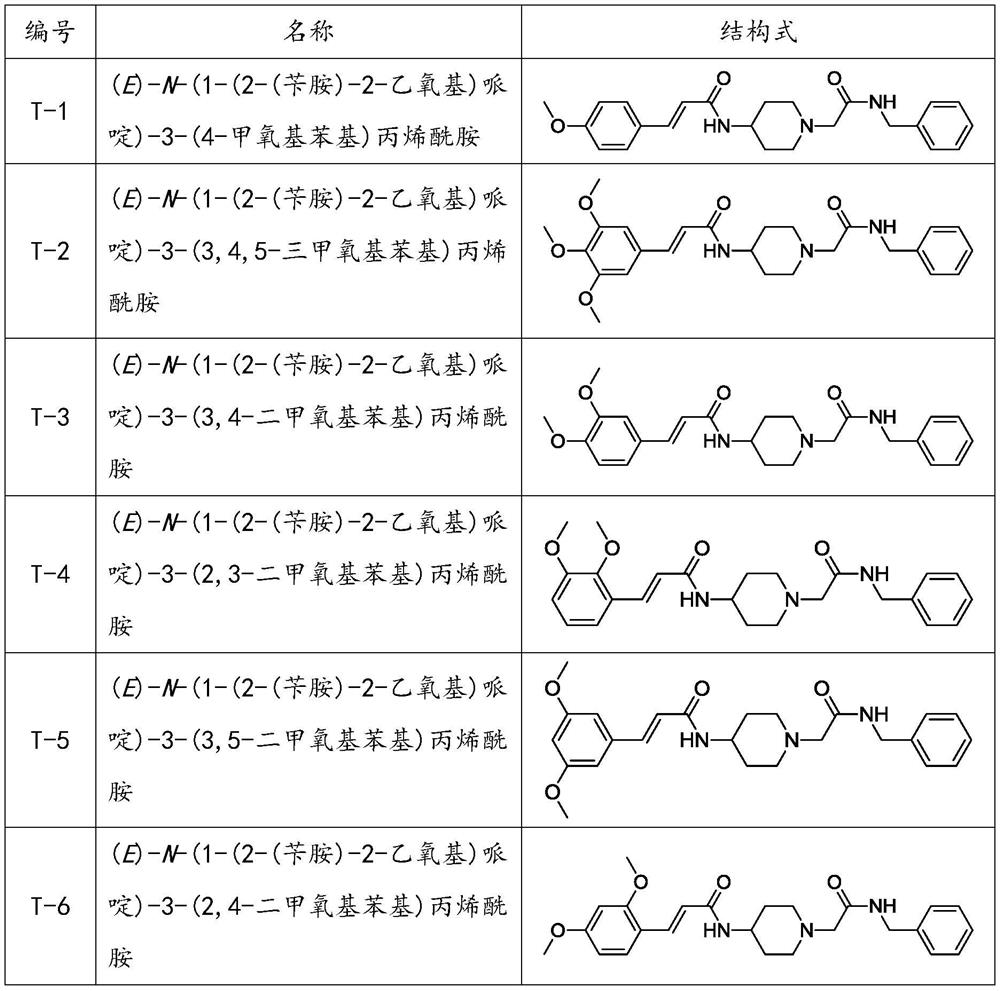
Acebenzylamine piperidinamide derivatives and their application as neuroprotective agents
A technology of acetobenzamide piperidinamide and derivatives, which is applied in the application field of brain neuroprotective agents, can solve the problems of high cardiotoxicity and weak activity, and achieves the effects of low acute toxicity and good curative effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] Example 1: (E)-N-(1-(2-(benzylamine)-2-ethoxy)piperidine)-3-(4-methoxyphenyl)acrylamide (T- 1) Preparation of its salt
[0067] Using 4-methoxycinnamic acid as raw material, according to the general synthetic method, 1.45 g of the target product was obtained with a yield of 85%.
[0068] ESI-MS[M+H] + m / z=408.2; 1 H NMR (400MHz, DMSO-d6) δppm: 10.17(s, 1H), 9.30(t, J=8.0Hz, 1H), 8.38(d, J=8.0Hz, 1H), 7.50(d, J=12.0Hz ,2H),7.41(s,1H),7.37~7.25(m,5H),6.98(d,J=8.0Hz,2H),6.53(d,J=16.0Hz,1H),4.36(d,J= 4.0Hz, 2H), 4.15~4.00(m, 3H), 3.79(s, 3H), 3.51(d, J=12.0Hz, 2H), 3.25~3.18(m, 2H), 2.08~1.98(m, 2H ),1.88~1.79(m,2H).
[0069] Preparation of compound T-1 hydrochloride
[0070] Compound T-1 (0.3g) and 5% hydrochloric acid aqueous solution (0.8mmol) were added to ethanol (10mL), refluxed and dissolved, and a white solid was precipitated by cooling, which was filtered to obtain 0.3g of white T-1 hydrochloride solid.
[0071] Preparation of compound T-1 mesylate
[007...
Embodiment 2
[0077] Example 2: (E)-N-(1-(2-(benzylamine)-2-ethoxy)piperidine)-3-(3,4,5-trimethoxyphenyl)propene Preparation of amides (T-2) and their salts
[0078] Using 3,4,5-trimethoxycinnamic acid as a raw material, and operating according to the general synthetic method, 1.45 g of the target product was obtained with a yield of 85%.
[0079] ESI-MS[M+H] + m / z=468.1; 1 H NMR (400MHz, DMSO-d6) δppm: 10.08(s, 1H), 9.25(t, J=8.0Hz, 1H), 8.35(d, J=8.0Hz, 1H), 7.40(s, 1H), 7.37 ~7.26(m, 5H), 6.90(d, J=4.0Hz, 2H), 6.61(d, J=16.0Hz, 1H), 4.36(d, J=4.0Hz, 2H), 4.06~3.99(m, 3H), 3.81(s, 6H), 3.68(s, 3H), 3.52~3.47(m, 2H), 3.25~3.11(m, 2H), 1.99(s, 2H), 1.86~1.78(m, 2H) .
[0080] Preparation of compound T-2 hydrobromic acid salt
[0081] Using compound T-2 (2.0 mmol) and 5% hydrobromic acid aqueous solution (2.1 mmol) as raw materials, the preparation method of compound T-1 hydrobromide was used to obtain 0.9 g of white T-2 hydrobromide solid.
Embodiment 3
[0082] Example 3: (E)-N-(1-(2-(benzylamine)-2-ethoxy)piperidine)-3-(3,4-dimethoxyphenyl)acryloyl Preparation of amine (T-3) and its salt
[0083] Using 3,4-dimethoxycinnamic acid as raw material, according to the general method, the target product was obtained 1.45g, and the yield was 85%.
[0084] ESI-MS[M+H] + m / z=438.2; 1H NMR (400MHz, DMSO-d6) δppm: 10.06(s, 1H), 9.23(d, J=8.0Hz, 1H), 8.29(d, J=8.0Hz, 1H), 7.38( d, J=8.0Hz, 2H), 7.35~7.25(m, 5H), 7.13(t, J=8.0Hz, 2H), 6.99(d, J=8.0Hz, 1H), 6.53(d, J=12.0 Hz, 1H), 4.36(d, J=4.0Hz, 2H), 4.12~3.88(m, 3H), 3.78(d, J=4.0Hz, 6H), 3.50(d, J=12.0Hz, 2H), 3.20(q, J=12.0Hz, 2H), 2.06~1.98(m, 2H), 1.85~1.77(m, 2H).
[0085] Preparation of Compound T-3 Fumarate
[0086] Using compound T-3 (2.3 mmol) and fumaric acid (2.4 mmol) as raw materials, the preparation method of compound T-1 hydrobromide was adopted to obtain 1.0 g of white solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com