Catalytic synthesis method of 6-difluorophenanthridine compound
A synthetic method, the technology of diflurphenidine, which is applied in the field of catalytic synthesis, can solve problems that have not been reported before, achieve good functional group compatibility, simple operation, and improve the effect of application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
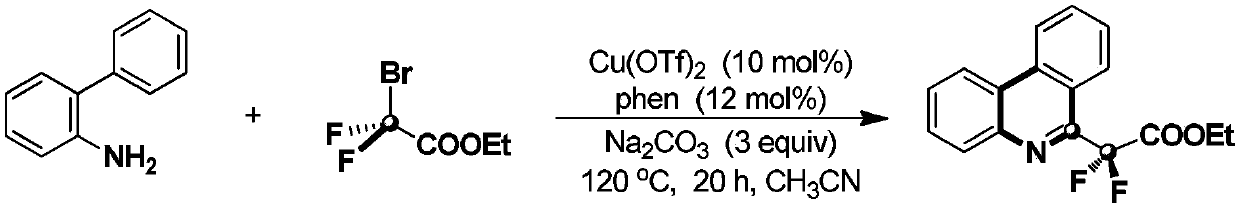
[0020] The reaction formula of this embodiment is as follows:
[0021]
[0022] (1) 0.2mmol o-aminobiphenyl, 0.6mmol ethyl bromodifluoroacetate, 0.02mmol Cu(OTf) 2 , 0.024mmol 1,10-phenanthroline, 0.6mmol sodium carbonate, and 2mL acetonitrile were placed in a pressure-resistant sealed reaction tube, filled with nitrogen and stirred in an oil bath at 120°C for reaction, followed by TLC and GC during the reaction To determine the specific reaction time, the reaction time is 20h;
[0023] (2) Cool the material obtained in step (1) to room temperature, add ethyl acetate to fully mix, filter, then wash with ethyl acetate, and finally combine the organic phases;
[0024] (3) spin dry the solvent in the organic phase of step (2) gained, rear use silica gel column purification product, then obtain described 1,6-difluoroacetate ethyl phenanthridine with eluent rinse, productive rate is 82 %, the eluent is a mixed solution of petroleum ether and ethyl acetate, and the volume ratio...
Embodiment 2
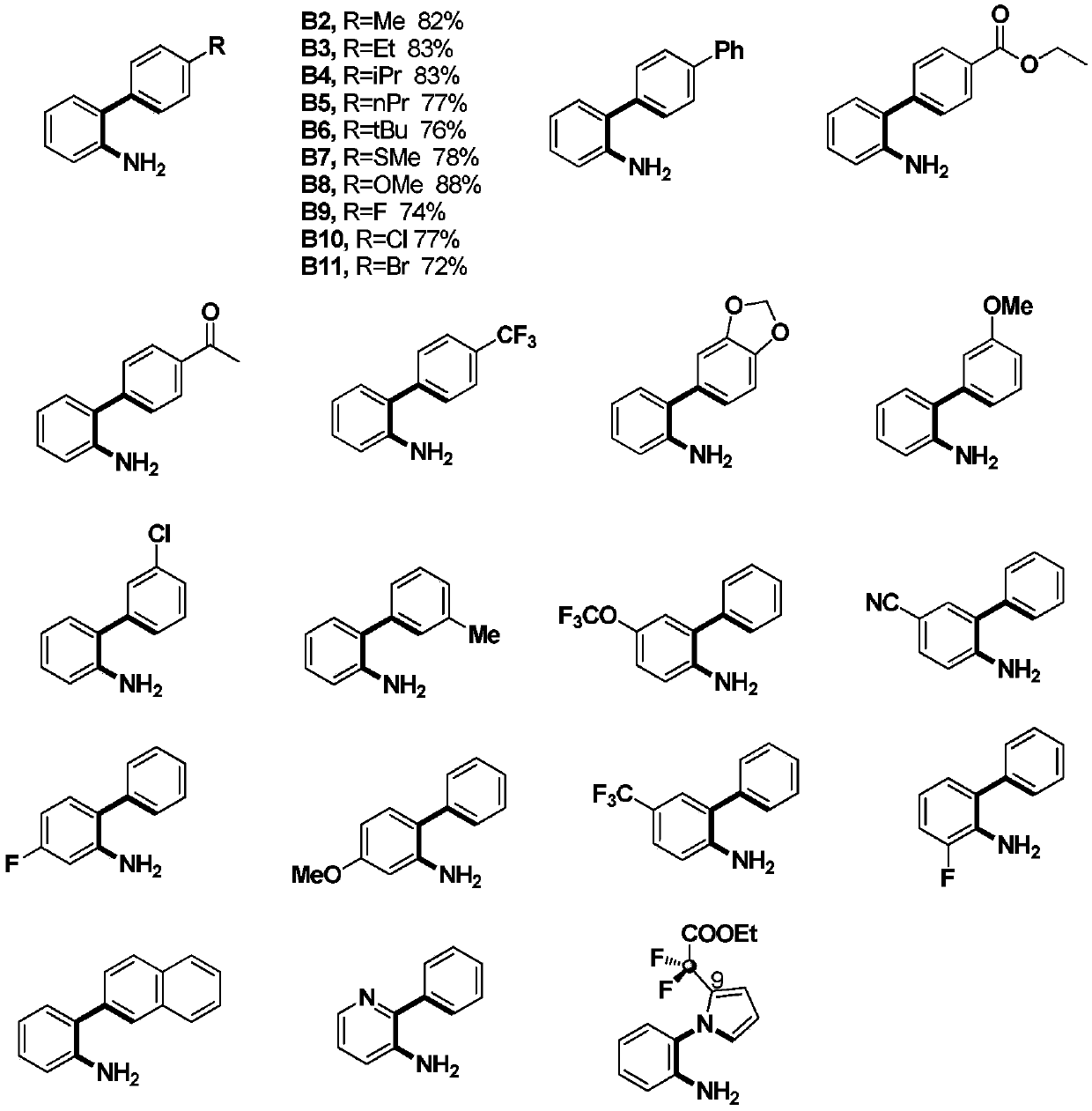
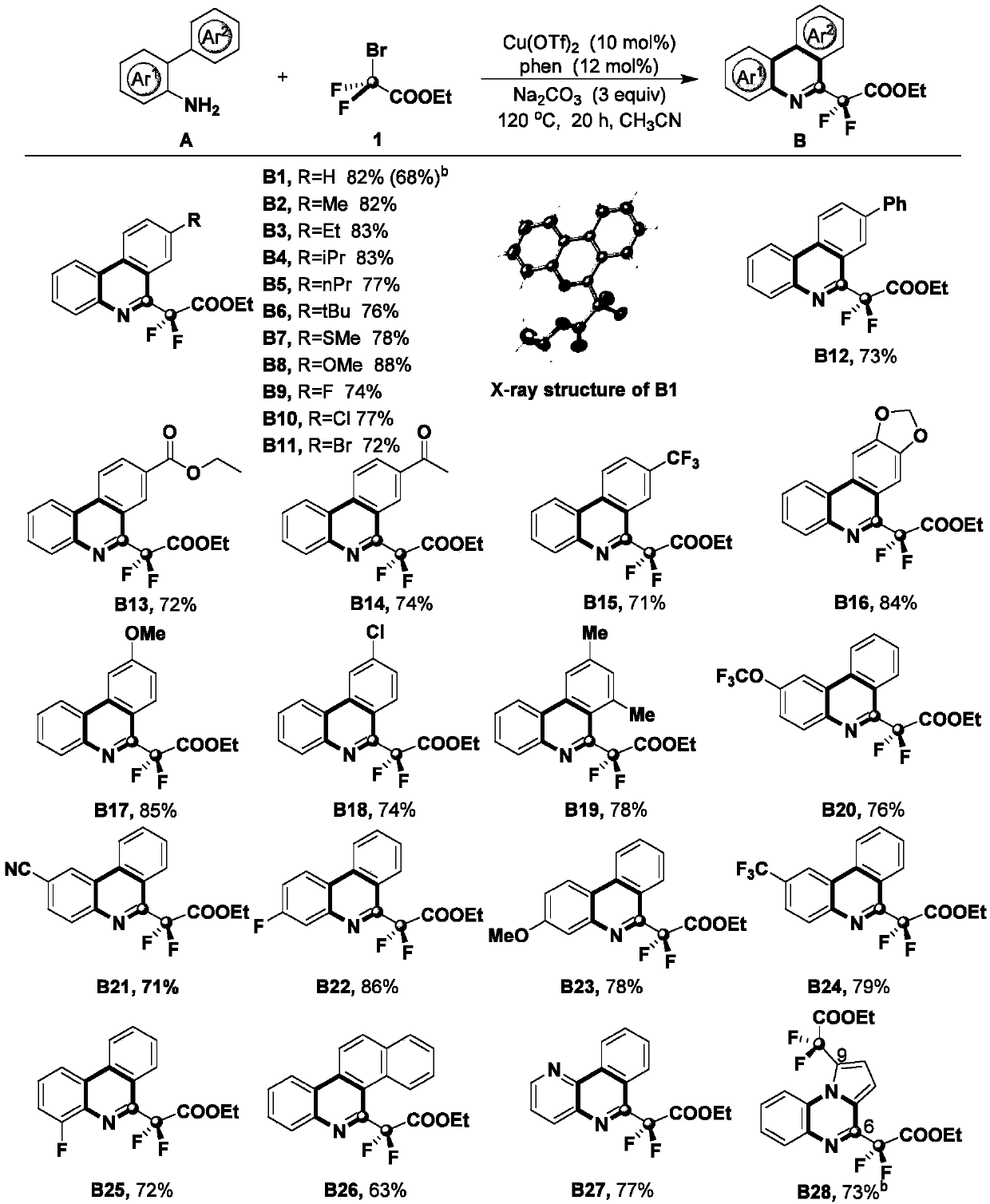
[0026] (1) 0.2mmol o-aminobiphenyl compounds, 0.6mmol ethyl bromodifluoroacetate, 0.02mmol Cu(OTf) 2 , 0.024mmol 1,10-phenanthroline, 0.6mmol sodium carbonate, and 2mL acetonitrile were placed in a pressure-resistant sealed reaction tube, filled with nitrogen and stirred in an oil bath at 120°C for reaction, followed by TLC and GC during the reaction To determine the specific reaction time, the reaction time is 20h;
[0027] The above-mentioned o-aminobiphenyl compounds are at least one of the following:
[0028]
[0029] (2) Cool the material obtained in step (1) to room temperature, add ethyl acetate to fully mix, filter, then wash with ethyl acetate, and finally combine the organic phases;
[0030] (3) spin dry the solvent in the organic phase of step (2) gained, after using silica gel column purification product, then obtain described 6-difluorophenanthidine compound with eluent rinse, this eluent is sherwood oil and ethyl acetate, and the volume ratio of petroleum et...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com