Method for photocatalytic synthesis of N-alkylphthalimide
A technology of alkyl phthalimide and alkenyl phthalimide is applied in the field of photocatalytic synthesis of N-alkyl phthalimide, which can solve the problem of poor versatility and universality , many by-products, low reaction efficiency and other problems, to achieve the effect of high promotion value and commercial value, less by-products, high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
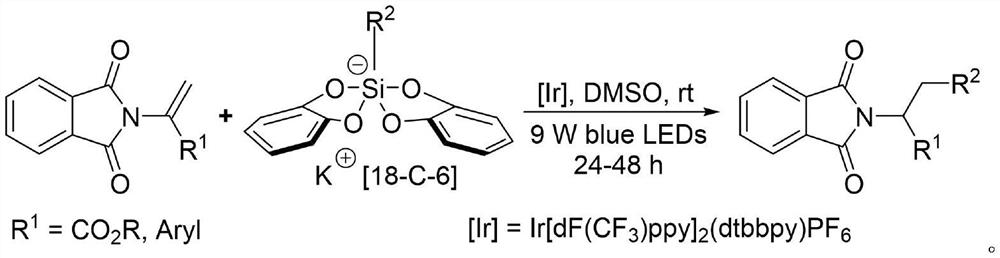
[0039] S1: Put the magneton into the reaction tube, add Ir[dF(CF 3 )ppy] 2 (dtbbpy)PF 6 (4.5 mg, 0.004 mmol, 0.02 equiv), 2-(N-phthalimide) ethyl acrylate (49.0 mg, 0.2 mmol, 1.0 equiv); put the reaction tube into the glove box, in the glove box Weigh chloropropyl bis(catechol) silicate-18-crown-6-potassium (249.8 mg, 0.4 mmol, 2.0 equiv);
[0040] S2: The reaction tube was plugged with a rubber stopper and taken out, dry dimethyl sulfoxide (6 mL) was added under nitrogen, and the rubber stopper was tightly sealed with a sealing film. The reaction tube was irradiated under a 9W blue LED lamp, and after stirring and reacting at room temperature for 24 hours, the light reaction was stopped, 6 mL of water was added to the reaction solution, and ethyl acetate (4×10 mL) was used for extraction;
[0041] S3: the organic phases were combined and washed with 5 mL of saturated brine, the organic phase was dried with anhydrous sodium sulfate and filtered, the filtrate was rotary evap...
Embodiment 2
[0046] S1: Put the magneton into the reaction tube, add Ir[dF(CF 3 )ppy] 2 (dtbbpy)PF 6 (4.5 mg, 0.004 mmol, 0.02 equiv), 2-(N-phthalimide) ethyl acrylate (49.0 mg, 0.2 mmol, 1.0 equiv); put the reaction tube into the glove box, in the glove box Weigh cyclohexyl bis(catechol) silicate-18-crown ether-6-potassium (252.0 mg, 0.4 mmol, 2.0 equiv);
[0047] S2: The reaction tube was plugged with a rubber stopper and taken out, dry dimethyl sulfoxide (6 mL) was added under nitrogen, and the rubber stopper was tightly sealed with a sealing film. The reaction tube was irradiated under a 9W blue LED lamp, and after stirring and reacting at room temperature for 24 hours, the light reaction was stopped, 6 mL of water was added to the reaction solution, and ethyl acetate (4×10 mL) was used for extraction;
[0048] S3: the organic phase was combined and washed with 5 mL of saturated brine, the organic phase was dried with anhydrous sodium sulfate and filtered, the filtrate was rotary ev...
Embodiment 3
[0053] S1: Put the magneton into the reaction tube, add Ir[dF(CF 3 )ppy] 2 (dtbbpy)PF 6 (4.5 mg, 0.004 mmol, 0.02 equiv), 1-(N-phthalimide) styrene (49.0 mg, 0.2 mmol, 1.0 equiv); put the reaction tube into the glove box, in the glove box Weigh ethyl bis(catechol) silicate-18-crown ether-6-potassium (230.4 mg, 0.4 mmol, 2.0 equiv);
[0054] S2: The reaction tube was plugged with a rubber stopper and taken out, dry dimethyl sulfoxide (6 mL) was added under nitrogen, and the rubber stopper was tightly sealed with a sealing film. The reaction tube was irradiated under a 9W blue LED lamp, and after stirring and reacting at room temperature for 24 hours, the light reaction was stopped, 6 mL of water was added to the reaction solution, and ethyl acetate (4×10 mL) was used for extraction;
[0055] S3: the organic phases were combined and washed with 5 mL of saturated brine, the organic phase was dried with anhydrous sodium sulfate and filtered, the filtrate was rotary evaporated t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com