Tumor immunotherapy method
A tumor, solid tumor technology, applied in the field of tumor treatment, can solve the problem of not showing a completely satisfactory effect on anti-tumor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
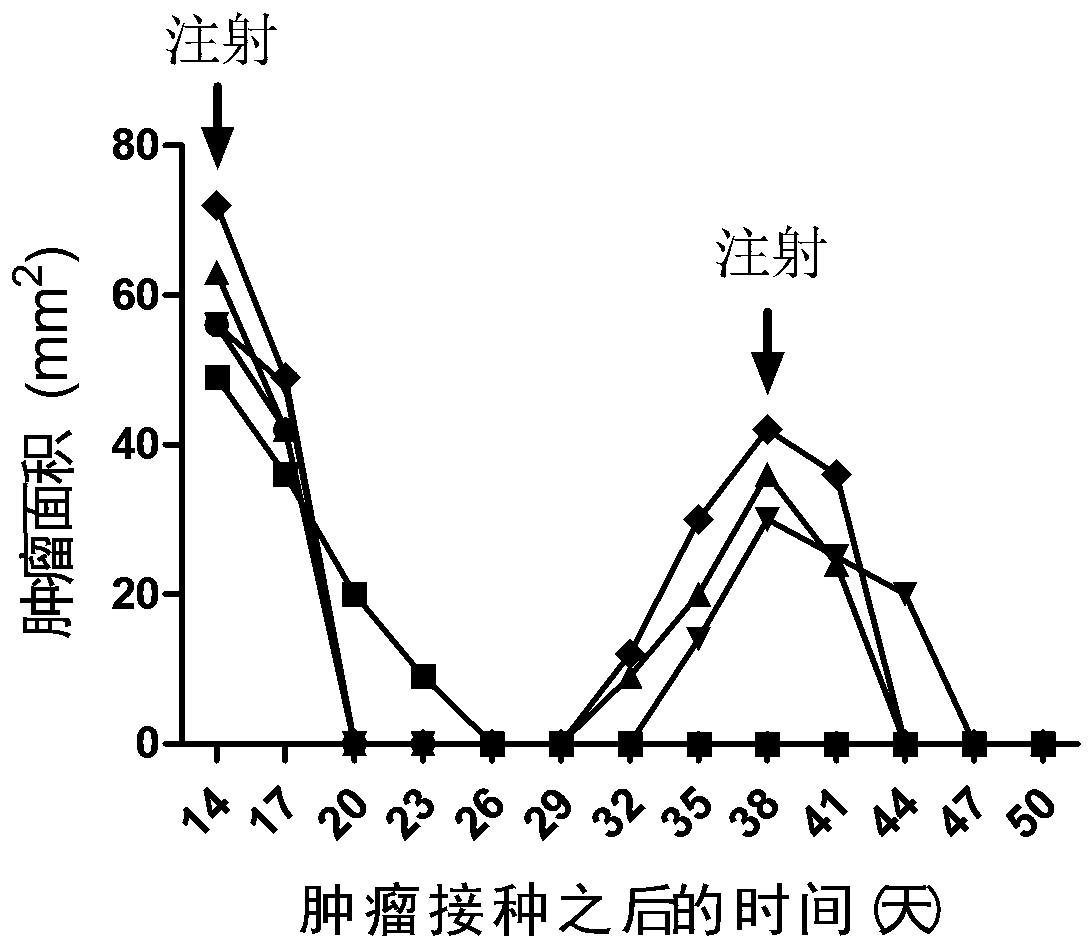
[0088] Example 1. Therapeutic effect of mIL12+mGMCSF+mIL2 chitosan quaternary ammonium salt solution on mouse melanoma
[0089] Digestion of cultured mouse melanoma cells (B16F10), 2 × 10 5 The cells were injected subcutaneously into the right side of the C57BL / 6 mouse body, and the treatment was started when the length of the tumor reached about 7-9 mm.
[0090] The cytokines mIL12, mGMCSF and mIL2 were dissolved in sterile water to make the final concentration of 20ng / µl, and 15µl of each was mixed to obtain a mixture of 45µl mIL12+mGMCSF+mIL2, and then pre-configured 3% chitosan was added. 45µl of sugar quaternary ammonium salt solution, mix by carefully pipetting with a pipette tip. The tumor-bearing mice were anesthetized by intraperitoneal injection of sodium pentobarbital, and then the prepared cytokine chitosan quaternary ammonium salt solution was drawn with a 29G insulin syringe, and slowly injected into the tumor. Spill of solution. After the injection, the mice ...
Embodiment 2
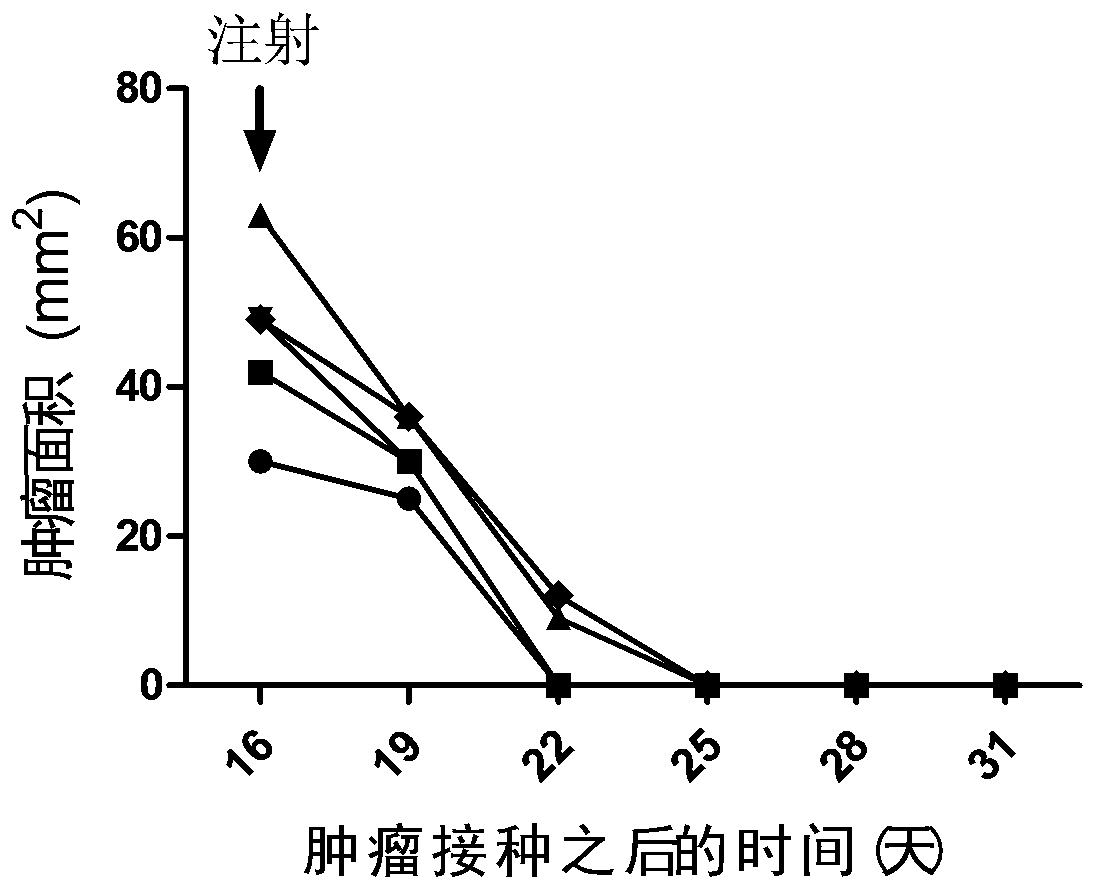
[0092] Example 2. Therapeutic effect of mIL12+mGMCSF+mIL2 chitosan quaternary ammonium salt solution on mouse colon cancer
[0093] Cultured mouse colon cancer cells (CT26) were digested and 5 × 10 5 The cells were injected subcutaneously on the right side of Balb / c mice, and the treatment was started when the length of the tumor reached about 7-9 mm.
[0094] The cytokines mIL12, mGMCSF and mIL2 were dissolved in sterile water to make the final concentration of 20ng / µl, and 15µl of each was mixed to obtain a mixture of 45µl mIL12+mGMCSF+mIL2, and then pre-configured 3% chitosan was added. 45µl of sugar quaternary ammonium salt solution, mix by carefully pipetting with a pipette tip. The tumor-bearing mice were anesthetized by intraperitoneal injection of sodium pentobarbital, and then the prepared cytokine chitosan quaternary ammonium salt solution was drawn with a 29G insulin syringe, and slowly injected into the tumor. Spill of solution. After the injection, the mice wer...
Embodiment 3
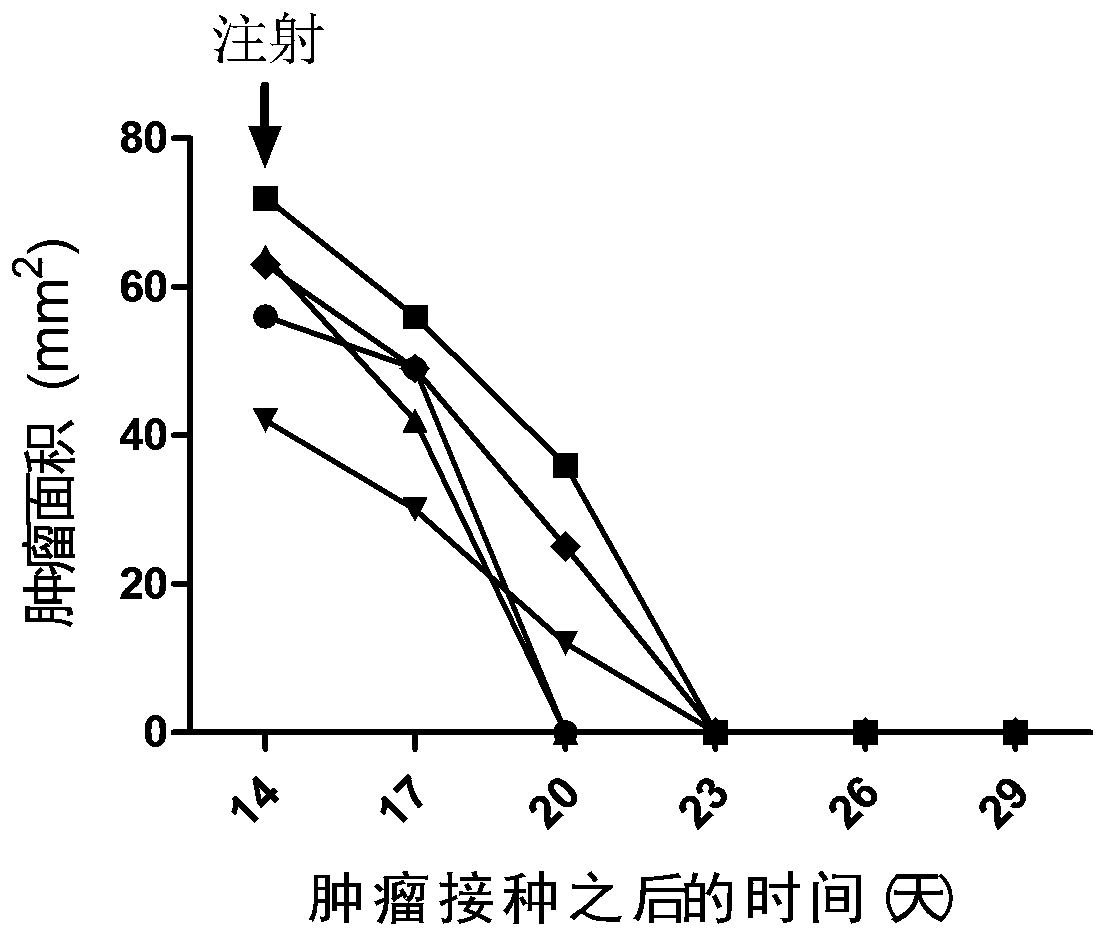
[0096] Example 3. Therapeutic effect of mIL12+mGMCSF+mIL2 chitosan quaternary ammonium salt solution on mouse breast cancer
[0097] Digestion of cultured mouse breast cancer cells (4T1), 2 × 10 5 The cells were injected subcutaneously on the right side of Balb / c mice, and the treatment was started when the length of the tumor reached about 7-9 mm.
[0098] Dissolve cytokines mIL12, mGMCSF and mIL2 with sterile water to make the final concentration of 20ng / µl, take 15µl of each and mix to obtain 45µl of mIL12+mGMCSF+mIL2 mixture, then add pre-configured 3% chitosan 45µl of sugar quaternary ammonium salt solution, mix by carefully pipetting with a pipette tip. The tumor-bearing mice were anesthetized by intraperitoneal injection of sodium pentobarbital, and then the prepared cytokine chitosan quaternary ammonium salt solution was drawn with a 29G insulin syringe, and slowly injected into the tumor. Spill of solution. After the injection, the mice were put back into the cage ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com