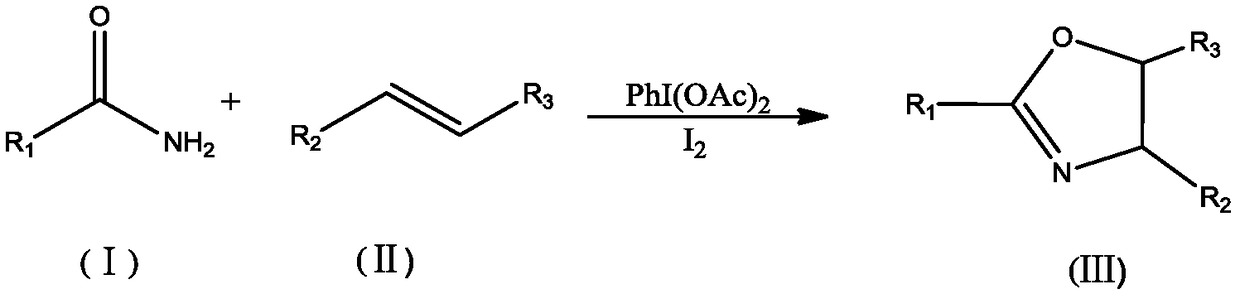
Method for synthesizing oxazoline derivative by use of amide
A technology for synthesizing oxazoline and derivatives, applied in the direction of organic chemistry and the like, can solve the problems of high risk and low yield, and achieve the effects of low toxicity, low price and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
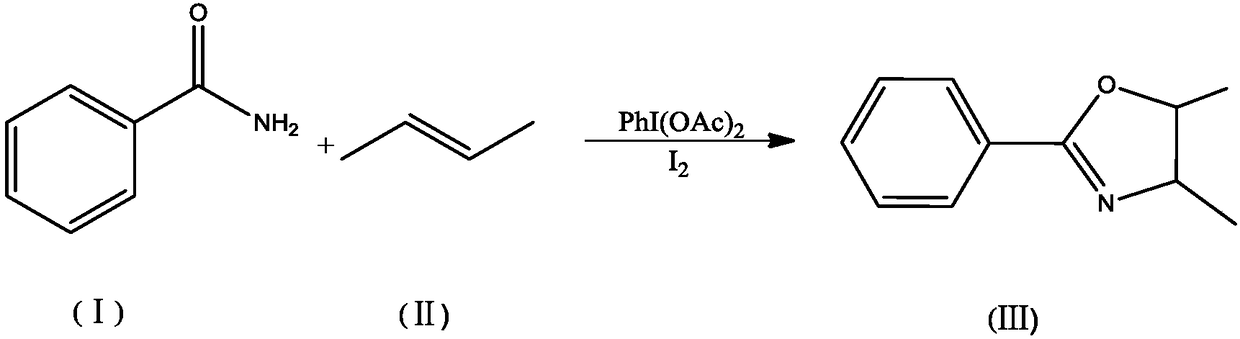
Embodiment 1
[0021] 121g of benzamide, 56g of 2-butene, 256g of I 2 and 322g PhI(OAc) 2 The catalyst combination was added to 500mL of chloroform, stirred at 30°C, irradiated with a high-pressure mercury lamp for 6 hours, washed with water, and the organic phase was separated and dried, and the organic phase was distilled off under reduced pressure to obtain a solid, namely The oxazoline derivative with the structure shown in formula (III) has a product yield of 92%.
[0022]
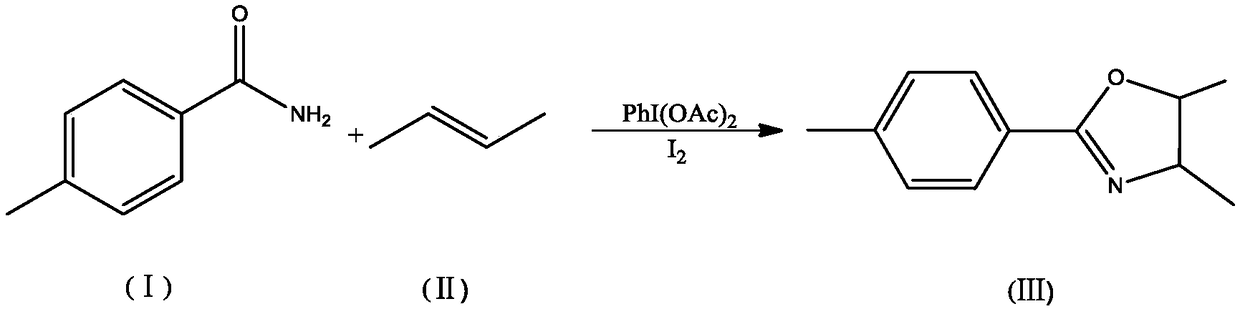
Embodiment 2
[0024] 135g of p-toluamide, 56g of 2-butene, 256g of I 2 and 322g PhI(OAc) 2 The catalyst combination was added into 500mL of chloroform, stirred at 40°C, irradiated with a high-pressure mercury lamp for 6 hours, washed with water, and the organic phase was separated and dried, and the organic phase was distilled off under reduced pressure to obtain a solid, namely The oxazoline derivative with the structure shown in formula (III) has a product yield of 91%.
[0025]
Embodiment 3
[0027] 135g of p-toluamide, 69g of 2-pentene, 256g of I 2 and 322g PhI(OAc) 2 The catalyst combination was added into 500mL of chloroform, stirred at 40°C, irradiated with a high-pressure mercury lamp for 6 hours, washed with water, and the organic phase was separated and dried, and the organic phase was distilled off under reduced pressure to obtain a solid, namely The oxazoline derivative with the structure shown in formula (III) has a product yield of 91%.
[0028]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com